
(2S,4R)-3-tert-butyl 4-methyl 2-tert-butyloxazolidine-3,4-dicarboxylate synthesis
- Product Name:(2S,4R)-3-tert-butyl 4-methyl 2-tert-butyloxazolidine-3,4-dicarboxylate
- CAS Number:380429-42-5
- Molecular formula:C14H25NO5
- Molecular Weight:287.35

24424-99-5
867 suppliers
$13.50/25G

257291-68-2
0 suppliers
inquiry

380429-42-5
17 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: di-tert-butyl dicarbonate;methyl (R)-2-tert-butyloxazolidine-4-carboxylate in methanol;n-heptane;tert-butyl methyl ether at 20 - 25; for 12 h;
Stage #2: with potassium carbonate in methanol;n-heptane;tert-butyl methyl ether;water at 20 - 25; for 12 h;
Steps:
2 Compound 30a:
D-Serine methyl ester hydrochloride 10 (100 g, 643 mmol, 1.0 equiv) was added to a suspension of sodium sulfate (183 g, 1290 mmol, 2.0 equiv) in toV-butyl methyl ether (400 mL). Methanol (50 mL) was then added followed by pivalaldehyde (77 mL, 690 mmol, 1.1 equiv) and triethylamine (90 mL, 643 mmol, 1.0 equiv). After stirring at 20-25 °C for 3 hours, n-heptane (500 mL) was added then the batch was stirred for an additional 15 minutes. The resulting suspension was then filtered, and the reactor and filter cake were washed with additional n- heptane (600 mL). To the combined filtrate was added a solution of di-tert-butyl dicarbonate (140 g, 643 mmol, 1.0 equiv) in n-heptane (50 mL). After stirring at 20-25 °C for 12 hours, a solution of potassium carbonate (300 mL of a 20% w/w solution in water) was added. After stirring at 20-25 °C for 12 hours, the aqueous phase was removed and A-methylpiperazine (25 mL, 225 mmol, 0.35 equiv) was added to the organic layer. After stirring at 20-25 °C for 1 hour the reaction was washed successively with 0.5 N HC1 (600 mL), 20% potassium carbonate (400 mL) and water (300 mL). The batch was then concentrated by vacuum to a clear oil. n-Heptane (500 mL) was then added and the batch was washed three times with water (3 c 300 mL). The resulting solution was then concentrated by vacuum to a neat oil. n-Heptane (300 mL) was then added and the batch was again concentrated to provide compound 30a as a neat oil. 1H NMR (400 MHz, DMSO-d6) d 4.91 (s, 1H), 4.71 (t, J = 6.8 Hz, 1H), 4.12 (d, J = 6.8 Hz, 1H), 3.67 (s, 3H), 1.40 (s, 9H), 0.86 (s, 9H).
References:
WO2020/206367,2020,A1 Location in patent:Paragraph 157
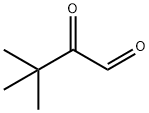
4480-47-1
14 suppliers
inquiry

380429-42-5
17 suppliers
inquiry
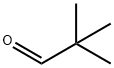
630-19-3
329 suppliers
$13.00/1g

380429-42-5
17 suppliers
inquiry