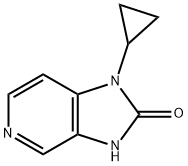
1-Cyclopropyl-1,3-dihydroimidazo[4,5-c]pyridine-2-one synthesis
- Product Name:1-Cyclopropyl-1,3-dihydroimidazo[4,5-c]pyridine-2-one
- CAS Number:380605-29-8
- Molecular formula:C9H9N3O
- Molecular Weight:175.19
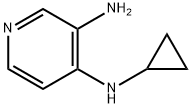
146950-68-7
59 suppliers
$157.00/250mg
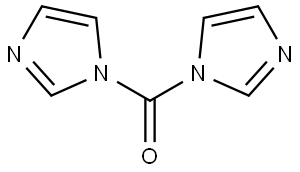
530-62-1
970 suppliers
$6.00/25g
![1-Cyclopropyl-1,3-dihydroimidazo[4,5-c]pyridine-2-one](/CAS2/GIF/380605-29-8.gif)
380605-29-8
52 suppliers
$137.00/100mg
Yield:380605-29-8 78.2%
Reaction Conditions:
in acetonitrile at 0 - 20; for 16 h;
Steps:
1
The synthesis of l-(cyclopropyl)-l,3-dihydro-2H-imidazo[4,5-c]pyridin-2-one (fragment CI) was done as shown in scheme 7.g 7 fragment C1; Scheme 7A round-bottom flask was charged with 3-nitro-4-chloropyridine (600 g, 3.8 mol), absolute EtOH (3L), diisopropylethylamine (DIPEA) (1320 mL, 7.6 mol) and cyclopropyl amine (432g, 7.6 mol). The resulting solution was refluxed for lOh. The reaction was cooled to 0°C, and the solid was collected by filtration. The filter cake was washed with cold ethanol (2x500 mL) to give compound 6. The mother liquor was concentrated and partitioned between water (1000 mL) and ethyl acetate (1000 mL). The aqueous layer was extracted with ethyl acetate (2x500mL), dried over MgS04, filtered, and concentrated to give a second batch of product (total: 650 g, 96%). A suspension of compound 6 (650g, 3.65mol) and 10% Pd/C (50% water; 163 g) in EtOH (7 L) was hydrogenated at 50 psi H2 for 16 h at room temperature. The suspension was filtered through Celite and concentrated. The residue was dried in vacuo to provide compound 7 (490 g, 90.6%). To a solution of compound 7 (490 g, 3.29 mol) in CH3CN (4 L) at 0°C was added carbonyldiimidazole (CDI) (559 g, 3.45 mol, 1.05eq.), and the resulting mixture was warmed to room temperature and stirred for 16 h at room temperature. The precipitate was collected by filtration and the solid was washed with cold CH3CN (2 X 1000 mL). The solid was dried in vacuo to give fragment CI . (450 g, 78.2%). m/z = 176 (M+H). 1H NMR (400 MHz, DMSO-d6) δ ppm 0.84 - 0.91 (m, 2 H), 0.98 - 1.06 (m, 2 H), 2.89 (tt, J=7.0, 3.5 Hz, 1 H), 7.18 (d, J=5.5 Hz, 1 H), 8.16 (s, 1 H), 8.19 (d, J=5.5 Hz, 1 H), 10.98 (br. s., 1 H).
References:
WO2012/80446,2012,A1 Location in patent:Page/Page column 18
31872-62-5
226 suppliers
$9.00/1g
![1-Cyclopropyl-1,3-dihydroimidazo[4,5-c]pyridine-2-one](/CAS2/GIF/380605-29-8.gif)
380605-29-8
52 suppliers
$137.00/100mg
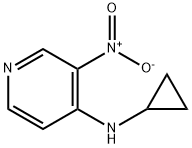
380605-28-7
49 suppliers
inquiry
![1-Cyclopropyl-1,3-dihydroimidazo[4,5-c]pyridine-2-one](/CAS2/GIF/380605-29-8.gif)
380605-29-8
52 suppliers
$137.00/100mg
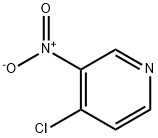
13091-23-1
342 suppliers
$14.14/1gm:
![1-Cyclopropyl-1,3-dihydroimidazo[4,5-c]pyridine-2-one](/CAS2/GIF/380605-29-8.gif)
380605-29-8
52 suppliers
$137.00/100mg
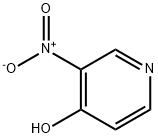
5435-54-1
368 suppliers
$19.00/25g
![1-Cyclopropyl-1,3-dihydroimidazo[4,5-c]pyridine-2-one](/CAS2/GIF/380605-29-8.gif)
380605-29-8
52 suppliers
$137.00/100mg