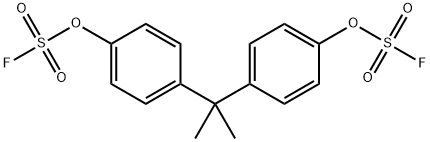
Fluorosulfuric acid, (1-methylethylidene)di-4,1-phenylene ester synthesis
- Product Name:Fluorosulfuric acid, (1-methylethylidene)di-4,1-phenylene ester
- CAS Number:38184-64-4
- Molecular formula:C15H14F2O6S2
- Molecular Weight:392.39
80-05-7
709 suppliers
$13.00/100g

38184-64-4
12 suppliers
inquiry
Yield:38184-64-4 100%
Reaction Conditions:
Stage #1: BPAwith triethylamine in dichloromethane at 20; for 0.166667 h;
Stage #2: with sulfuryl fluoride in dichloromethane at 20;Sealed tube;
Steps:
2.A.i 2A(i). Preparation of propane-2,2-diylbis(4,l-phenylene) difluorosulfonate (BPA- ()S()2F) from WO 2014/089078 to Dong et al.
2A(i). Preparation of propane-2,2-diylbis(4,l-phenylene) difluorosulfonate (BPA- ()S()2F) from WO 2014/089078 to Dong et al. A 2-liter single-neck round-bottom flask was charged with bisphenol A (114.9 g, 0.5 mol), CH2CI2 (DCM; 1 L) and triethylamine (Et3N; 174 mL, 1.25 mol, 2.5 equivalents). The mixture was stirred at room temperature for 10 minutes (min). The reaction flask was then sealed with a septum, the atmosphere above the solution was removed with gentle vacuum, and SO2F2 gas (sulfuryl fluoride, VIKA E) was introduced by needle from a balloon filled with the gas. For large scale reactions such as this, depletion of the sulfuryl fluoride from the balloon is easily observed, and more reagent is introduced with a fresh balloon when required. For small scale reactions, SO2F2 is used in excess. These reactions can be easily followed by thin layer chromatography (TLC). The reaction mixture was vigorously stirred at room temperature for about 2 to 4 hours, monitoring by GC-MS and TLC. After completion, the solvent was removed by rotary evaporation, the residue was dissolved in ethyl acetate (EtOAc; 1 L), and the solution was washed with IN HC1 (2 x 500 mL) and brine (2 x 500 mL). The organic phase was dried over anhydrous Na2S04 and concentrated. The resulting solid was dried under high vacuum at about 60 °C overnight to afford BPA-OS02F as a white crystalline solid in quantitative yield (typically about 197 g, 100% yield). Melting point (MP) 48-49 °C. 1H NMR (400 MHz, CDC13) δ 7.34- 7.32 (m, 2H), 7.28-7.26 (m, 2H), 1.72 (s, 3H); 13C NMR (101 MHz, CDC13) δ 150.4, 148.2, 128.7, 120.5, 42.9, 28.4, 30.7; 19F NMR (376 MHz, CDC13) δ +37.0; GC-MS (tR): 7.2 min; EI- MS (m/z): 392 [M]+.
References:
WO2016/209920,2016,A1 Location in patent:Page/Page column 36