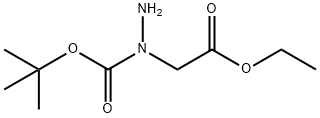
Hydrazinecarboxylic acid, 1-(2-ethoxy-2-oxoethyl)-, 1,1-dimethylethyl ester synthesis
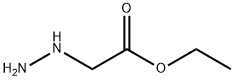
- Product Name:Hydrazinecarboxylic acid, 1-(2-ethoxy-2-oxoethyl)-, 1,1-dimethylethyl ester
- CAS Number:3842-40-8
- Molecular formula:C9H18N2O4
- Molecular Weight:218.25
Yield:3842-40-8 95%
Reaction Conditions:
with 4-methyl-morpholine in ethanol;water at 20;Cooling with ice;
Steps:
I
(N-tert-Butoxycarbonyl-hydrazino)-acetic acid ethyl ester A mixture of hydrazino-acetic acid ethyl ester HCl salt (4.04 g, 26.13 mmol), di-tert-butyl dicarbonate (5.70 g, 26.13 mmol), and N-methylmorpholine (2.87 g, 28.42 mmol) was stirred in ethanol (25 mL) and water (25 mL) under ice bath. The reaction was warmed to room temperature and stirred for 3 h. The mixture was quenched with saturated ammonium chloride (100 mL) and the aqueous solution was extracted twice with 100 mL of diethyl ether. The ether layer was washed with water and dried with magnesium sulfate. Solvents were removed to afford (N-tert-butoxycarbonyl-hydrazino)-acetic acid ethyl ester (5.40 g, 95% yield) as colorless oil. 1H NMR (DMSO-d6) δ (ppm) 4.55 (s, 2H), 4.03-4.20 (m, 2H), 4.01 (s, 2H), 1.38 (s, 9H), 1.20 (t, J=7.1 Hz, 3H).
References:
US2011/112147,2011,A1 Location in patent:Page/Page column 10
13139-12-3
173 suppliers
$5.00/1g
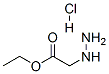
6945-92-2
194 suppliers
$6.00/1g
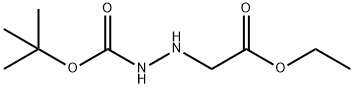
3842-39-5
1 suppliers
inquiry

3842-40-8
3 suppliers
inquiry