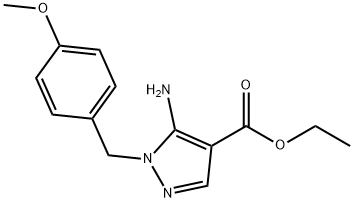
ethyl 5-amino-1-(4-methoxybenzyl)-1H-pyrazole-4-carboxylate synthesis
- Product Name:ethyl 5-amino-1-(4-methoxybenzyl)-1H-pyrazole-4-carboxylate
- CAS Number:384835-92-1
- Molecular formula:C14H17N3O3
- Molecular Weight:275.3
Yield:384835-92-1 92%
Reaction Conditions:
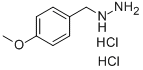
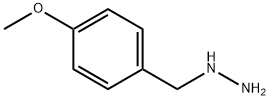
Stage #1: (4-methoxybenzyl)hydrazine hydrochloridewith triethylamine in ethanol at 20; for 0.5 h;
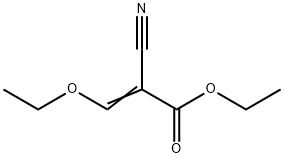
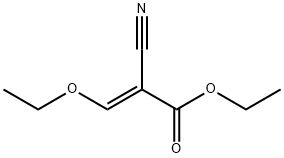
Stage #2: ethyl (ethoxymethylene)cyanoacetate in ethanol;Reflux;
Steps:
1 Step 1: Synthesis of ethyl 5-amino-1-(4-methoxybenzyl)-1H-pyrazole-4-carboxylate
(4-methoxybenzyl)hydrazine hydrochloride (100.0 g, 0.53 mol) was dissolved in absolute ethanol (1.6 L), and triethylamine (81.0 g, 0.80 mol) was added. The reaction solution was stirred at room temperature for 30 minutes, and ethyl (ethoxymethylene)cyanoacetate (98.0 g, 0.58 mol) was added. The reaction mixture was stirred under reflux overnight, and concentrated to remove ethanol. Water (500 mL) was added to the solid residue, which was then extracted with ethyl acetate (2 500 mL). The organic phases were combined, washed with a saturated brine (300 mL), dried with anhydrous sodium sulfate, and filtered with suction. The filtrate was concentrated to obtain ethyl 5-amino-1-(4-methoxybenzyl)-1H-pyrazole-4-carboxylate (135.0 g, yield: 92%). MS m/z (ESI): 276 [M+H]+.
References:
EP3964518,2022,A1 Location in patent:Paragraph 0161-0162