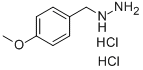
(4-METHOXYBENZYL)HYDRAZINE DIHYDROCHLORIDE synthesis
- Product Name:(4-METHOXYBENZYL)HYDRAZINE DIHYDROCHLORIDE
- CAS Number:2011-48-5
- Molecular formula:C8H14Cl2N2O
- Molecular Weight:225.12
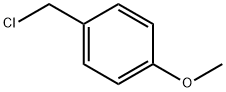
824-94-2

2011-48-5
Step 1: Synthesis of (4-methoxybenzyl)hydrazine hydrochloride Hydrazine hydrate (40 g, 0.80 mol) and anhydrous ethanol (280 mL) were added to a 500 mL three-necked round bottom flask. A solution of anhydrous ethanol (30 mL) of 4-methoxybenzyl chloride (12.5 g, 0.080 mol) was slowly added dropwise to the above mixture at room temperature. The reaction mixture was heated to 90 °C and stirred for 2 hours. Upon completion of the reaction, the mixture was concentrated under reduced pressure to remove the solvent and the residue was subsequently redissolved in anhydrous ethanol (150 mL). The solution was cooled to 0 °C and acidified by slowly adding 5 M hydrochloric acid (120 mL). The precipitated white solid precipitate was collected by filtration and dried to give 8.72 g of the target product (4-methoxybenzyl)hydrazine hydrochloride in 72% yield. Mass spectrometry analysis showed m/z: 153(M+H)+.

824-94-2
449 suppliers
$24.19/5G

2011-48-5
133 suppliers
inquiry
Yield:2011-48-5 80%
Reaction Conditions:
Stage #1: p-methoxybenzyl chloridewith hydrazine hydrate in methanol at 0 - 20; for 2 h;
Stage #2: with hydrogenchloride in 1,4-dioxane; for 3 h;
Steps:
D Step-D:
Synthesis of (4-methoxybenzyl)hydrazine hydrochloride (Intermediate C)
To a stirred solution of hydrazinehydrate (3.19 mL, 63.8 mmol) in methanol (5 mL) was added 1-(chloromethyl)-4-methoxybenzene (1 g, 6.38 mmol) at 0°C, and the mixture was stirred at room temperature for 2 hours.
The progress of the reaction was monitored by TLC.
After the reaction completed, the reaction mixture was distilled off under reduced pressure and extracted with ether.
The combined organic extracts were washed with water and brine, dried over anhydrous sodium sulfate, and distilled off under reduced pressure.
The crude compound was taken in dioxane (5 mL), 4.0M dioxane hydrochloric acid (2 mL) was added thereto, and the mixture was stirred at room temperature for 3 hours.
The precipitated solid was filtered, washed with ether and dried to obtain Compound C (1 g, 80%).
The same batch was repeated with the scales of 1 g, 50 g, 100 g and 350 g.
1H NMR (400 MHz, DMSO-d6): δ 7.68 (brs, 2H), 7.38 (dd, 2H), 6.85 (dd, 2H), 3.98 (s, 2H), 3.78 (s, 3H).
References:
EP3640253,2020,A1 Location in patent:Paragraph 0094-0095