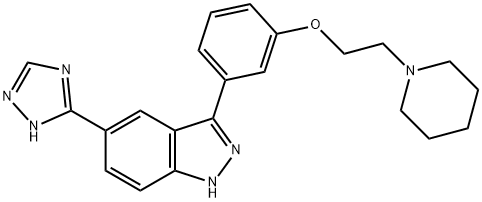
3-[3-[2-(1-Piperidinyl)ethoxy]phenyl]-5-(1H-1,2,4-triazol-5-yl)-1H-indazole synthesis
- Product Name:3-[3-[2-(1-Piperidinyl)ethoxy]phenyl]-5-(1H-1,2,4-triazol-5-yl)-1H-indazole
- CAS Number:395104-30-0
- Molecular formula:C22H24N6O
- Molecular Weight:388.47
![1H-Indazole-5-carboxamide, 3-[3-[2-(1-piperidinyl)ethoxy]phenyl]-](/CAS/20210305/GIF/395106-69-1.gif)
395106-69-1
0 suppliers
inquiry
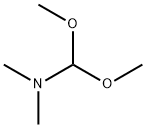
4637-24-5
631 suppliers
$5.00/25g
![3-[3-[2-(1-Piperidinyl)ethoxy]phenyl]-5-(1H-1,2,4-triazol-5-yl)-1H-indazole](/CAS2/GIF/395104-30-0.gif)
395104-30-0
95 suppliers
$93.00/1mg
Yield:395104-30-0 90%
Reaction Conditions:
Stage #1: 3-(3-(2-(piperidin-1-yl)ethoxy)phenyl)-1H-indazole-5-carboxamide;N,N-dimethyl-formamide dimethyl acetal in tetrahydrofuran;Industry scale;
Stage #2: with acetic acid;hydrazine in tetrahydrofuran at 25 - 67;Product distribution / selectivity;Industry scale;
Steps:
6.2.2.6
A 640 L reactor was cleaned and purged with N2. 3-(3-(2-(piperidin-1-yl)ethoxy)phenyl)-1H-indazole-5-carboxamide was suspended in THF and dimethylformamid-dimethylacetal was added at about 22° C. The reaction mixture was stirred at about 64° C. for about three hours resulting in a clear solution, which was cooled to about 16° C. overnight. Conversion was estimated to be >99% by HPLC. At a jacket temperature of about 60° C. and reduced pressure, 350 L of solvent were distilled. Dichloro-methane and water were added at an inner temperature of about 27° C. and the layers were separated. Approximately 166 L of the organic layer were distilled (about 60° C. jacket temperature, reduced pressure). TBME was added and the distillation was continued (about 115 L of distillate). A second portion of TBME was added. The resulting suspension was stirred at about 53 to about 55° C. for about I hour, cooled to about 1° C., and stirred for about 15 minutes at this temperature. The precipitated intermediate was collected by filtration and washed with TBME. The intermediate was dried on the nutsch filter dryer in a stream of nitrogen for about 1 h and finally at about 50° C. jacket temperature and reduced pressure overnight (about 12 h) (38.841 kg isolated). Hydrazine monohydrate was added to acetic acid and THF at about 25 to about 30° C. over about 20 minutes. 38.841 kg of the intermediate were added over about 20 min at about 42 to about 49° C. The reaction mixture was stirred at about 67° C. inner temperature (reflux) for about 4.5 h. Conversion was estimated to be 97% by HPLC. Stirring was continued for about 1.5 h at about 67° C. inner temperature. Conversion was estimated to again be 97% by HPLC. Stirring was continued overnight (about 13 h) at about 67° C. inner temperature. Conversion was estimated to be 99.5% by HPLC. At an internal temperature of about 25° C., 25% NH3-solution was added (over about 2.5 h) to adjust the pH to about 10. The layers were separated and the aqueous phase was extracted twice with THF. Reactor und feed tanks were rinsed with water and afterwards with inline-filtered THF. The nutsch filter dryer (without filter cloth) was rinsed with inline-filtered acetonitrile. Afterwards a new filter cloth was installed. The clean reactor was charged with the combined, inline-filtered organic layers (355 L). At a jacket temperature of about 60° C. and reduced pressure, about 162 L of solvent were distilled. The product solution was stirred for about 5 days at about 20° C. Inline-filtered acetonitrile was added to the solution and the resulting suspension was heated. At about 60° C. a clear solution was formed. The clear solution was cooled to about 52° C. inner temperature and seeded with a suspension of 1-(5-(1H-1,2,4-triazol-5-yl)(1H-indazol-3-yl))-3-(2-piperidylethoxy)benzene in inline-filtered acetonitrile. At a jacket temperature of about 60° C. and reduced pressure, the distillation was started. The distillation was carried out with an inner temperature controlled at about 40° C. Upon starting the distillation, a suspension formed immediately. About 360 L of solvent were distilled and inline-filtered acetonitrile was added in parallel to maintain a constant volume of the mixture. 1.6 mol % THF was estimated to be present by NMR. Additional inline-filtered acetonitrile was added and the distillation was restarted. About 40 L of solvent were distilled. 1.2 mol % THF was estimated to be present by NMR. Additional inline-filtered acetonitrile was added and about 40 L of solvent were removed by distillation. 0.8 mol % THF was estimated to be present by NMR. The suspension was stirred at about 52° C. inner temperature overnight (about 10 h), cooled to about 20° C. over about one hour, and stirred at this temperature for about one hour. Crude product was collected by filtration and the filter cake was washed with inline-filtered acetonitrile in two portions. The product was dried on the nutsch filter dryer in a stream of nitrogen for about 1 h and finally at about 50° C. jacket temperature and reduced pressure for about 28 h (34.071 kg; 90% yield; 99.68% purity by HPLC).
References:
US2006/258706,2006,A1 Location in patent:Page/Page column 18; 20
3040-44-6
255 suppliers
$20.00/25g

395104-16-2
8 suppliers
inquiry
![3-[3-[2-(1-Piperidinyl)ethoxy]phenyl]-5-(1H-1,2,4-triazol-5-yl)-1H-indazole](/CAS2/GIF/395104-30-0.gif)
395104-30-0
95 suppliers
$93.00/1mg