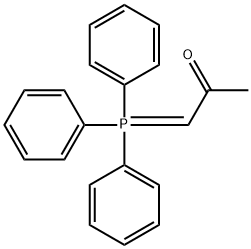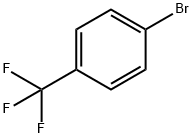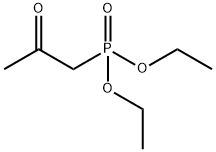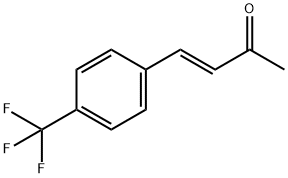
(3E)-4-[4-(trifluoroMethyl)phenyl]but-3-en-2-one synthesis
- Product Name:(3E)-4-[4-(trifluoroMethyl)phenyl]but-3-en-2-one
- CAS Number:115665-92-4
- Molecular formula:C11H9F3O
- Molecular Weight:214.18
Yield: 92%
Reaction Conditions:
in dichloromethane for 15 h;Wittig Reaction;
Steps:
38
E-4-(4-(Trifluoromethyl)phenyl)but-3-en-2-one. 4-(Trifluoromethyl)benzaldehyde (25.Og, 144 mmol)(commercially available from 3B Scientific Corporation Product List (Order Number 3B4-3644)) was taken up in 500 mL of DCM. l-Triphenylphosphoranylidene-2-propanone (48.0 g, 151 mmol) was added. After 5 hours, an additional 3 g of the Wittig reagent was added. The mixture was stirred for 10 hours. The solvent was removed under reduced pressure, and the residue was triturated with 500 mL of 5% EtOAc/hexanes. The mixture was filtered, removing a large amount Of P(O)Ph3. The residue was taken up in 300 mL of 2.5% EtOAc/hexanes and filtered through a pad of silica. The mixture was concentrated under reduced pressure, and the residue was found to be (E)-4-(4-(trifluoromethyl)phenyl)but-3-en-2- one (28.3 g, 92.0% yield). 1H NMR (400 MHz, CDCl3) δ ppm 7.68 - 7.60 (m, 3H), 7.52 (d, J= 16.43 Hz, 1H), 6.78 (d, J = 16.4 Hz, 1H), 2.41 (s, 3H).
References:
AMGEN INC. WO2009/11871, 2009, A2 Location in patent:Page/Page column 174
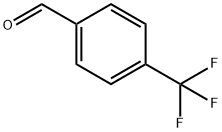
455-19-6
413 suppliers
$6.00/5g
123-42-2
598 suppliers
$19.00/100ml
![(3E)-4-[4-(trifluoroMethyl)phenyl]but-3-en-2-one](/CAS/GIF/115665-92-4.gif)
115665-92-4
22 suppliers
inquiry