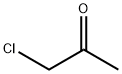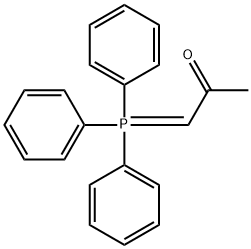
(ACETYLMETHYLENE)TRIPHENYLPHOSPHORANE synthesis
- Product Name:(ACETYLMETHYLENE)TRIPHENYLPHOSPHORANE
- CAS Number:1439-36-7
- Molecular formula:C21H19OP
- Molecular Weight:318.35

Yield:1439-36-7 23.3%
Reaction Conditions:
in chloroform at 70; for 12 h;Inert atmosphere;
Steps:
1 [00162] Step 1: l-(triphenylphosphoranylidene)propan-2-one
[00162] Step 1: l-(triphenylphosphoranylidene)propan-2-one: A solution of 1- chloropropan-2-one (50 g, 540.4 mmol) in chloroform (150 mL) was added drop wise to a solution of triphenylphosphine (141.72 g, 540.4 mmol) in chloroform (150 mL) under nitrogen. The mixture was stirred at 70 °C for 12 h, and the resulting phosphonium salt was filtered. The precipitate was washed with ethyl acetate and dried under vacuum. The dried phosphonium salt was suspended in a mixture of water (250 mL) and methanol (250 mL), and the mixture was stirred for 1 h. Aqueous sodium hydroxide (2.00 M) was added to the mixture until a pH between 7 and 8 was reached. The mixture was then stirred vigorously for 1 h. The phosphorane precipitate was filtered and washed with water. After drying in vacuum, the phosphorane was recrystallized from ethyl acetate and dried under vacuum to afford l-(triphenylphosphoranylidene)propan-2-one (40.00 g, 23.3%) as a white solid.
References:
CONSTELLATION PHARMACEUTICALS, INC.;ALBRECHT, Brian, K.;AUDIA, James, Edmund;DAKIN, Les, A.;DUPLESSIS, Martin;GEHLING, Victor, S.;HARMANGE, Jean-Christophe;NASVESCHUK, Christopher, G.;VASWANI, Rishi, G. WO2015/23915, 2015, A1 Location in patent:Paragraph 00161; 00162
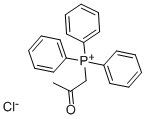
1235-21-8
162 suppliers
$10.00/1g

1439-36-7
214 suppliers
$8.00/5g
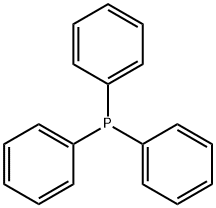
603-35-0
733 suppliers
$5.00/5 g

1439-36-7
214 suppliers
$8.00/5g
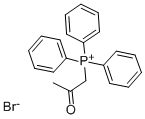
2236-01-3
44 suppliers
$54.20/5g

1439-36-7
214 suppliers
$8.00/5g

603-35-0
733 suppliers
$5.00/5 g
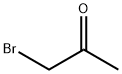
598-31-2
124 suppliers
$60.00/50mg

1439-36-7
214 suppliers
$8.00/5g