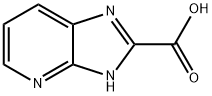
3H-imidazo[4,5-b]pyridine-2-carboxylic acid synthesis
- Product Name:3H-imidazo[4,5-b]pyridine-2-carboxylic acid
- CAS Number:97640-15-8
- Molecular formula:C7H5N3O2
- Molecular Weight:163.13
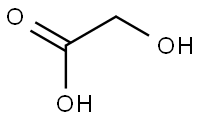
![3H-imidazo[4,5-b]pyridin-2-ylmethanol](/CAS/20200331/GIF/24638-20-8.gif)
24638-20-8
![3H-imidazo[4,5-b]pyridine-2-carboxylic acid](/CAS/20200331/GIF/97640-15-8.gif)
97640-15-8
Step 1004b: Preparation of 3H-imidazo[4,5-b]pyridine-2-carboxylic acid. To a boiling aqueous solution of KMnO4 (0.38 g, 2.4 mmol) (8 mL), an aqueous solution of 3H-imidazo[4,5-b]pyridine-2-methanol (0.20 g, 1.4 mmol) and Na2CO3 (0.19 g, 1.5 mmol) (6 mL) was slowly added. The reaction mixture was heated to reflux for 4 hours. The reaction mixture was filtered while hot and the filtrate was cooled to room temperature and the pH was adjusted with concentrated hydrochloric acid to 2. The precipitate precipitated was collected by vacuum filtration to afford 3H-imidazo[4,5-b]pyridine-2-carboxylic acid in 100% yield. Mass spectrometry (MS) analysis results: C7H5N3O2, m/z 164 (M + H)+.
![3H-imidazo[4,5-b]pyridin-2-ylmethanol](/CAS/20200331/GIF/24638-20-8.gif)
24638-20-8
26 suppliers
$45.00/50mg
![3H-imidazo[4,5-b]pyridine-2-carboxylic acid](/CAS/20200331/GIF/97640-15-8.gif)
97640-15-8
37 suppliers
$60.00/5mg
Yield:97640-15-8 100%
Reaction Conditions:
with hydrogenchloride;potassium permanganate;sodium carbonate in water;
Steps:
b N-[(3R)-1-Azabicyclo[2.2.2]oct-3-yl]-3H-imidazo[4,5-b]pyridine-2-carboxamide Hydrochloride
Step 1004b: Preparation of 3H-imidazo[4,5-b]pyridine-2-carboxylic Acid. To a boiling solution of KMnO4 (0.38 g, 2.4 mmol) in water (8 mL), a boiling solution of 3H-imidazo[4,5-b]pyridin-2-ylmethanol (0.20 g, 1.4 mmol) and Na2CO3 (0.19 g, 1.5 mmol) in water (6 mL) is added. The resulting mixture is heated at reflux for 4 hr. The hot mixture is filtered, the filtrate is cooled to rt, and the pH adjusted to 2 using conc. HCl. The resulting precipitate is collected by vacuum filtration to afford 3H-imidazo[4,5-b]pyridine-2-carboxylic acid in 100% yield: MS for C7H5N3O2: m/z 164 (M+H)+.
References:
US2003/73707,2003,A1
![1H-Imidazo[4,5-b]pyridine-2-methanol, -alpha--methyl- (9CI)](/CAS/GIF/250651-52-6.gif)
250651-52-6
5 suppliers
inquiry
![3H-imidazo[4,5-b]pyridine-2-carboxylic acid](/CAS/20200331/GIF/97640-15-8.gif)
97640-15-8
37 suppliers
$60.00/5mg
452-58-4
471 suppliers
$14.00/1g
![3H-imidazo[4,5-b]pyridine-2-carboxylic acid](/CAS/20200331/GIF/97640-15-8.gif)
97640-15-8
37 suppliers
$60.00/5mg