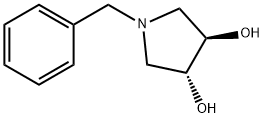
(3R,4R)-(-)-1-BENZYL-3,4-PYRROLIDINDIOL synthesis
- Product Name:(3R,4R)-(-)-1-BENZYL-3,4-PYRROLIDINDIOL
- CAS Number:163439-82-5
- Molecular formula:C11H15NO2
- Molecular Weight:193.24
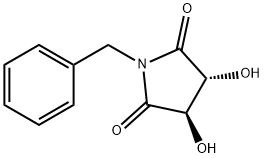
75172-31-5

163439-82-5
General procedure for the synthesis of (3R,4R)-1-benzyl-3,4-dihydroxypyrrolidine-2,5-dione from (3R,4R)-1-benzyl-3,4-pyrrolidinediol: a tetrahydrofuran solution of borane (1.0 M, 17.19 mL, 180.99 mmol) was added slowly and dropwise to a tetrahydrofuran solution (1.0 M, 17.19 mL, 180.99 mmol) of (3R,4R)-1-benzyl-3,4-dihydroxy pyrrolidine-2,5-dione (10.0 g, 45.24 mmol) in a solution of tetrahydrofuran (200 mL). After dropwise addition, the reaction mixture was slowly heated to 70°C and kept at this temperature for 3 hours. Upon completion of the reaction, the mixture was cooled to 0°C and the reaction was quenched by the dropwise addition of methanol (50 mL), noting that a strong gas release occurs at this point. Subsequently, the mixture was slowly warmed to room temperature and stirring was continued for 1 hour. The reaction mixture was concentrated under vacuum and the crude product was purified by silica gel column chromatography using a linear gradient elution of ethyl acetate in petroleum ether to afford 7.73 g (88.5% yield) of the yellow crystalline product (3R,4R)-1-benzyl-3,4-pyrrolidinediol. Specific optical rotation (MeOH, 0.5%): +22.800°; 1H NMR (400 MHz, DMSO-d6) δ 7.51-7.49 (2H, m), 7.39-7.37 (3H, m), 5.45 (1H, d, J = 4.4 Hz, OH), 5.20 (1H, d, J = 5.2 Hz, OH), 4.15- 4.11 (1H, m), 4.04-3.94 (2H, m), 3.90-3.85 (1H, m), 3.48-3.43 (1H, m), 3.10-3.06 (1H, m), 2.97-2.93 (1H, m), 2.89-2.86 (1H, m); 13C NMR (100 MHz, DMSO-d6) δ 132.86, 128.97, 128.51, 127.73, 76.27, 65.47, 63.24; LC-MS (ESI) m/z Calculated value C11H15NO2 [M + H]+: 193.11, measured value: 194.20.
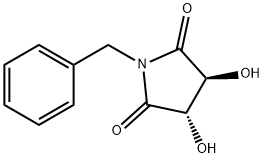
187032-53-7
38 suppliers
$92.00/100mg

163439-82-5
128 suppliers
$59.00/1g
Yield:163439-82-5 99%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran; for 12 h;Heating;
References:
Bridgeman, Eve;Cavill, Julie L.;Schofield, Daniel J.;Wilkins, Derek S.;Tomkinson, Nicholas C. O. [Tetrahedron Letters,2005,vol. 46,# 49,p. 8521 - 8524]

75172-31-5
59 suppliers
$45.00/10mg

163439-82-5
128 suppliers
$59.00/1g
109-63-7
406 suppliers
$10.60/1gm:

187032-53-7
38 suppliers
$92.00/100mg

163439-82-5
128 suppliers
$59.00/1g

100-46-9
501 suppliers
$5.00/5G

163439-82-5
128 suppliers
$59.00/1g