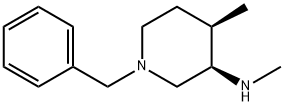
(3R,4R)-1-Benzyl-N,4-dimethylpiperidin-3-amine synthesis
- Product Name:(3R,4R)-1-Benzyl-N,4-dimethylpiperidin-3-amine
- CAS Number:477600-70-7
- Molecular formula:C14H22N2
- Molecular Weight:218.34
1615709-89-1

477600-70-7
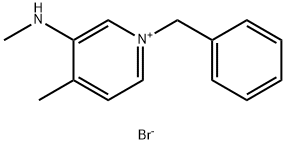
The general procedure for the synthesis of (3R,4R)-1-benzyl-4-methyl-3-(methylamino)pyridin-1-ium bromide as a raw material for the synthesis of (3R,4R)-1-benzyl-4-methyl-3-methylamino-piperidines was as follows: 1-benzyl-3-methylamino-4-methyl-pyridinium bromide (10 g, 34.1 mmol) was placed in a 250 ml reaction flask, ethanol (100 g) was added, and a temperature of below 30°C and stirring was started. Sodium borohydride (3.87 g, 102.3 mmol) was added slowly and after addition, the reaction was continued with stirring for 16 h. The reaction was monitored by HPLC until the content of raw material was below 1%. Subsequently, 2M HCl solution was slowly added dropwise to the reaction system until no bubbles were generated. The reaction solution was concentrated to one third of the original volume under reduced pressure. It was extracted twice with dichloromethane, the organic phases were combined and concentrated under reduced pressure to remove the solvent. To the resulting crude product, ethanol (40 g) was added and 2M hydrochloric acid ethanol solution (20 ml) was slowly added dropwise at a temperature below 30 °C. A solid precipitated. After dropwise addition, stirring was continued for 1 h. Subsequently, filtration was carried out and the filter cake was dried under reduced pressure. The final white solid product (3R,4R)-1-benzyl-4-methyl-3-methylamino-piperidine (6.9 g, 23.8 mmol) was obtained in 70% yield.

1615709-89-1
19 suppliers
inquiry

477600-70-7
218 suppliers
$49.00/1g
Yield:477600-70-7 70%
Reaction Conditions:
Stage #1: 1-benzyl-4-methyl-3-(methylamino)pyridinium bromidewith sodium tetrahydroborate at 30; for 16 h;
Stage #2: with hydrogenchloride;ethanol at 30;
Steps:
1 Preparation Example 1: Synthesis of Compound 6 and Preparation of Intermediate
Add 1-benzyl-3-methylamino-4-methyl-pyridine bromide 15 (10 g, 34.1 mmol) to a 250 ml reaction vial, add ethanol (100 g), and start stirring at a temperature below 30 °C. Add sodium borohydride (3.87 g, to the reaction solution,102.3 mmol), after the addition was completed, the reaction solution was stirred for 16 hours, and the compound 15 was detected by HPLC to be less than 1%.2M HCl was added dropwise to the reaction solution, no bubbles were formed in the reaction system, and the reaction solution was concentrated to a one-third volume under reduced pressure.It was extracted twice with dichloromethane and the organic phases were combined and concentrated under reduced pressure to basic solvent free.To the crude product, ethanol (40 g) was added, and 2M hydrochloric acid ethanol (20 ml) was added dropwise at a temperature below 30 °C, and a solid precipitated. After the addition, stirring was continued for 1 hour, suction filtration, and the filter cake was dried under reduced pressure.Obtained a white product (6.9 g, 23.8 mmol),The yield was 70%.
References:
CN108610279,2018,A Location in patent:Paragraph 0014; 0016
384338-23-2
79 suppliers
$75.00/1g

477600-70-7
218 suppliers
$49.00/1g
![Carbamic acid, N-[(3R,4R)-4-methyl-1-(phenylmethyl)-3-piperidinyl]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/1092578-34-1.gif)
1092578-34-1
0 suppliers
inquiry

477600-70-7
218 suppliers
$49.00/1g