
4-(2,2-Difluoroethoxy)Benzaldehyde synthesis
- Product Name:4-(2,2-Difluoroethoxy)Benzaldehyde
- CAS Number:128140-72-7
- Molecular formula:C9H8F2O2
- Molecular Weight:186.16
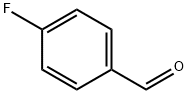
459-57-4
603 suppliers
$6.00/25g

128140-72-7
21 suppliers
$45.00/10mg
Yield:128140-72-7 77%
Reaction Conditions:
Stage #1: difluoroethanolwith sodium hydride in N,N-dimethyl-formamide at 0; for 0.166667 h;
Stage #2: 4-fluorobenzaldehyde in N,N-dimethyl-formamide at 0 - 20; for 4.33333 h;
Steps:
4.4a
With reference to the document (J. Med. Chem., (1994), 37, 3977-3985), sodium hydride (3.36 g, 55%, 77.1 mmol) was added to a solution of DMF (100 mL) containing 2,2-difluoroethanol (5.75 g, 70.1 mmol) over 5 minutes under nitrogen gas flow under ice-cooling. The mixture was stirred at the same temperature for 10 minutes, and then a solution of DMF (40 mL) containing 4-fluorobenzaldehyde (9.56 g, 77.0 mmol) was added dropwise thereto over 5 minutes. The mixture was stirred at room temperature for 4 hours and was then poured into ice water (500 mL). The resulting mixture was extracted with ether : hexane (300 mL, 1:1, v/v) three times. The extracted organic layer was washed with water (300 mL) three times and then with saturated brine, and was dried over anhydrous magnesium sulfate. Then, the solvent was evaporated to give a crude product. An ether/hexane mixture solution (20 mL, 1:10, v/v) was added to the crude product, and the supernatant fluid was removed. This procedure was repeated four times in total to wash the crystals to give 10.1 g of the title compound (colorless crystalline solid, yield: 77%). 1H-nuclear magnetic resonance spectrum (500 MHz, CDCl3) δ ppm: 9.92 (1H, s), 7.87 (2H, d, J=8Hz), 7.04 (2H, d, J=8Hz), 6.13 (1H, tt, J=55Hz, 4Hz), 4.27 (2H, td, J=13Hz, 4Hz).
References:
EP1880720,2008,A1 Location in patent:Page/Page column 53
22042-71-3
144 suppliers
$14.00/1g

128140-72-7
21 suppliers
$45.00/10mg