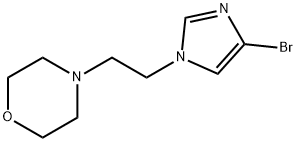
4-[2-(4-bromo-1H-imidazol-1-yl)ethyl]morpholine synthesis
- Product Name:4-[2-(4-bromo-1H-imidazol-1-yl)ethyl]morpholine
- CAS Number:877399-20-7
- Molecular formula:C9H14BrN3O
- Molecular Weight:260.13
Yield:877399-20-7 42%
Reaction Conditions:
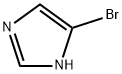
Stage #1: 4-bromo-1 H-imidazolewith caesium carbonate in N,N-dimethyl-formamide; for 0.5 h;
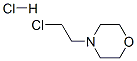
Stage #2: 4-(2-chloroethyl)morpholine hydrochride in N,N-dimethyl-formamide at 50;
Steps:
48
General Procedure 48 A mixture of 4-bromo-imidazole (217 mg, 1.48 mmol) and cesium carbonate (875 mg, 2.69 mmol) in dimethylformamide (5 mL) was stirred for 30 minutes. 4-(2-Chloro-ethyl)-morpholine hydrochloride (250 mg, 1.34 mmol) was added and the mixture was heated to 50° C. After heating overnight the reaction was concentrated by rotary evaporation. The residue was suspended in a mixture of dichloromethane and methanol and filtered. The filtrate was concentrated by rotary evaporation. The residue was purified by silica gel chromatography using gradient elution of dichloromethane, methanol to afford 4-[2-(4-Bromo-imidazol-1-yl)-ethyl]-morpholine (148 mg, 42%).
References:
US2006/46991,2006,A1 Location in patent:Page/Page column 50

879488-40-1
3 suppliers
inquiry
![4-[2-(4-bromo-1H-imidazol-1-yl)ethyl]morpholine](/CAS/20180702/GIF/877399-20-7.gif)
877399-20-7
4 suppliers
inquiry
![1H-Imidazole, 4-bromo-1-[2-(triphenylmethoxy)ethyl]-](/CAS/20210305/GIF/879488-22-9.gif)
879488-22-9
0 suppliers
inquiry
![4-[2-(4-bromo-1H-imidazol-1-yl)ethyl]morpholine](/CAS/20180702/GIF/877399-20-7.gif)
877399-20-7
4 suppliers
inquiry