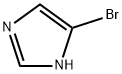
4-Bromo-1H-imidazole synthesis
- Product Name:4-Bromo-1H-imidazole
- CAS Number:2302-25-2
- Molecular formula:C3H3BrN2
- Molecular Weight:146.97
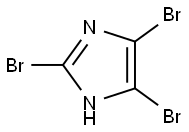
2034-22-2

2302-25-2
General procedure for the synthesis of 4-bromo-1H-imidazole from 2,4,5-tribromoimidazole: 2,4,5-tribromoimidazole (49 g, 161 mmol), sodium sulfite (101.5 g, 806 mmol), and water (500 mL) were added to a single-necked flask, and the reaction was stirred for 6 h at 110 °C. The reaction was carried out with the addition of ethyl acetate. After completion of the reaction, ethyl acetate was added for extraction, the organic layers were combined and dried with anhydrous sodium sulfate. The ethyl acetate was removed by concentration under reduced pressure to afford the target product 4-bromo-1H-imidazole (20.5 g, 89% yield).

2034-22-2
192 suppliers
$9.00/10g

2302-25-2
255 suppliers
$6.00/1g
Yield:2302-25-2 89%
Reaction Conditions:
with sodium sulfite in water at 110; for 6 h;
Steps:
1.3 (3) Synthesis of 4-bromoimidazole (Intermediate 6)
To a single-necked flask was added 2,4,5-tribromoimidazole (49 g, 161 mmol)Sodium sulfite (101.5 g, 806 mmol)And water (500 ml) were added and stirred at 110 ° C for 6 h,Ethyl acetate was added,The organic layers were combined and dried over anhydrous sodium sulfate,Rotate the ethyl acetate to give compound 6 (20.5 g, yield 89%)
References:
Hinova Pharmaceuticals Inc.;Fan, Lei;Chen, Ke;Li, Xinghai;Chen, Yuanwei CN106256830, 2016, A Location in patent:Paragraph 0044; 0052; 0053; 0054

288-32-4
1039 suppliers
$5.00/25g
2302-30-9
88 suppliers
$50.00/50mg

2302-25-2
255 suppliers
$6.00/1g

2302-30-9
88 suppliers
$50.00/50mg

2302-25-2
255 suppliers
$6.00/1g
64591-03-3
70 suppliers
$109.00/250 mg

2302-25-2
255 suppliers
$6.00/1g

288-32-4
1039 suppliers
$5.00/25g

2302-30-9
88 suppliers
$50.00/50mg

2034-22-2
192 suppliers
$9.00/10g

2302-25-2
255 suppliers
$6.00/1g