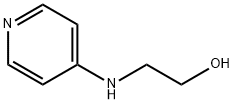
4-(2-HYDROXYETHYLAMINO)-PYRIDINE synthesis
- Product Name:4-(2-HYDROXYETHYLAMINO)-PYRIDINE
- CAS Number:192130-06-6
- Molecular formula:C7H10N2O
- Molecular Weight:138.17
626-61-9
49 suppliers
$365.00/500mg

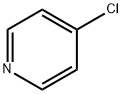
141-43-5
862 suppliers
$9.00/10g

192130-06-6
23 suppliers
$45.00/100mg
Yield: 100%
Reaction Conditions:
at 110; for 3 h;
Steps:
11 4-((2-Hydroxyethyl)amino)pyridine (49)
4-((2-Hydroxyethyl)amino)pyridine (49) A mixture of 4-chloropyridine (500mg; 4.41mmol) in ethanolamine (2.6mL; 44mmol) was stirred at 1 10°C for 3h. The solvent was removed and the residue was purified by silica gel flash chromatography using a linear gradient of ethyl acetate (0- 100% AcOEt) in cyclohexane to afford 49 as a white powder (607mg; 4.40mmol; quantitative yield). 1H NMR (500MHz, DMSO) δ 8.00 (d, J=6.1Hz, 1H, Hc5), 6.49 (m, 3H, Hc4 and HNH), 4.77 (brs, 1H, HOH), 3.53 (t, J=6.0Hz, 2H, Hcl), 3.13 (q, J=5.9Hz, 2H, Hc2). 13C NMR (125MHz, DMSO) δ: 154.1 (Cc3), 149.7(Cc5), 107.5 (Cc4), 59.7 (Ccl), 40.6 (Cc2). HRMS-ESI (m/z) calculated for C7Hi0N2NaO [M+Na] : 161.0685; found: 161.0650.
References:
PIERRE FABRE MEDICAMENT;CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE (CNRS);HALBY, Ludovic;ARIMONDO, Paola Barbara WO2015/40169, 2015, A1 Location in patent:Page/Page column 64-65