4-(2-Hydroxynaphthalen-1-yl)benzaldehyde synthesis
- Product Name:4-(2-Hydroxynaphthalen-1-yl)benzaldehyde
- CAS Number:56432-18-9
- Molecular formula:C17H12O
- Molecular Weight:232.28
Yield:56432-18-9 99%
Reaction Conditions:
with tetrakis(triphenylphosphine) palladium(0);potassium carbonate in ethanol;water;toluene; for 24 h;Inert atmosphere;Reflux;
Steps:
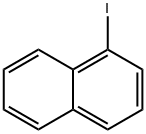
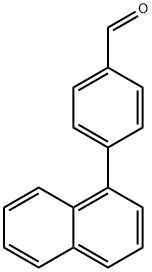
1 Synthesis of 4-(1-naphthyl)benzaldehyde:
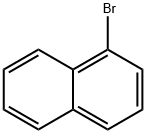
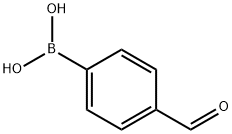
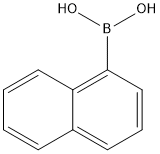
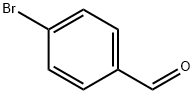
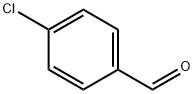
To 100 mL of toluene (40 mL), 1-bromonaphthalene (1.8 g, 8.69 mmol), 4-formylbenzeneboronic acid (1.3 g, 8.69 mmol) and potassium carbonate (4.81 g, 34.77 mmol) were sequentially added to a three-necked flask of a mixture of ethanol (8 mL) and water (16 mL). Dissolved by magnetic stirring. Nitrogen gas was again vigorously bubbled in the solution for 5 min, tetrakis(triphenylphosphine)palladium (0.3 g, 0.26 mmol) was added and the mixture was vigorously bubbled with nitrogen for 5 min. The reaction mixture was refluxed for 24 hours under a nitrogen atmosphere. After the reaction was completed, the reaction mixture was cooled to room temperature, extracted three times with ethyl acetate, washed three times with water and dried over anhydrous sodium sulfate. The solvent was evaporated to give a white solid powder (2 g). The yield was 99%.
References:
CN109574942,2019,A Location in patent:Paragraph 0016; 0017
1351445-39-0
0 suppliers
inquiry
128376-64-7
189 suppliers
$5.00/250mg

56432-18-9
17 suppliers
inquiry