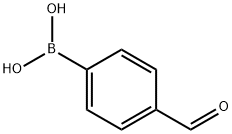
4-Formylphenylboronic acid synthesis
- Product Name:4-Formylphenylboronic acid
- CAS Number:87199-17-5
- Molecular formula:C7H7BO3
- Molecular Weight:149.94
![Boronic acid, [4-(2-propenyl)phenyl]-](/CAS/20180529/GIF/186497-79-0.gif)
186497-79-0
1 suppliers
inquiry

87199-17-5
433 suppliers
$8.00/5g
Yield:87199-17-5 88%
Reaction Conditions:
with Triethoxysilane;iron(III) chloride in methanol; for 12 h;Reflux;
Steps:
2 Example 2Synthesis of Compound 2:
In the air, 25 mL of the reaction flask was sequentially added with ferric chloride (0.05 mmol), alkene 1b (0.5 mmol), triethoxySilane (1.5 mmol) and methanol (2.0 mL). After mixing at room temperature, the reaction mixture was reacted under reflux for 12 h. anti-It should be completed and directly chromatographed to give a yield of 88%.
References:
CN109796293,2019,A Location in patent:Paragraph 0039-0041; 0149
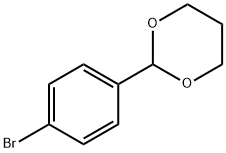
61568-51-2
19 suppliers
inquiry

87199-17-5
433 suppliers
$8.00/5g
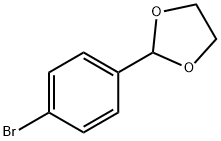
10602-01-4
112 suppliers
$22.00/1g

5419-55-6
388 suppliers
$12.00/5g

87199-17-5
433 suppliers
$8.00/5g

10602-01-4
112 suppliers
$22.00/1g

87199-17-5
433 suppliers
$8.00/5g

10602-01-4
112 suppliers
$22.00/1g

121-43-7
382 suppliers
$14.00/25mL

87199-17-5
433 suppliers
$8.00/5g