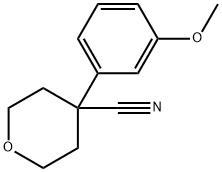
4-(3-Methoxyphenyl)oxane-4-carbonitrile synthesis
- Product Name:4-(3-Methoxyphenyl)oxane-4-carbonitrile
- CAS Number:179420-73-6
- Molecular formula:C13H15NO2
- Molecular Weight:217.26
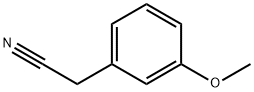
19924-43-7
269 suppliers
$9.00/10g
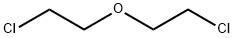
111-44-4
338 suppliers
$13.00/50g

179420-73-6
14 suppliers
$323.00/1g
Yield:179420-73-6 28%
Reaction Conditions:
Stage #1: 3-methoxyphenylacetonitrilewith sodium hydride in DMF (N,N-dimethyl-formamide) at 0 - 20; for 0.5 h;
Stage #2: 3-oxa-1,5-dichloropentane in DMF (N,N-dimethyl-formamide) at 0 - 20; for 2.33333 h;
Steps:
4.1
Intermediate 4: 3-{4-[(dimethylamino)methyl]tetrahydro-pyran-4-yl}phenol [] Step 1:; 3-Methoxyphenylacetonitrile (10g, 67.9mmol) in DMF (50ml) was added slowly to a suspension of NaH (5.04g, 150mmol) in DMF (50ml) at 0°C under N2. The reaction was allowed to warm to room temperature and stirred for 30mns. The reaction was cooled to 0°C and bis(2-chloroethyl)ether (10.7g, 74.7mmol) in DMF (100ml) was added dropwise over 80 min. The reaction was allowed to warm to room temperature and stirred for 1 hour. The reaction was quenched with water (500ml) and extracted with ethyl acetate (3 x 100ml). The combined organic extracts were washed with brine (3 x 50ml), dried over MgSO4, filtered, washed with ethyl acetate and concentrated in vacuo. The crude mixture was purified by column chromatography, eluting with heptane increasing the polarity to heptane:ethyl acetate (90 :10), to give 4-(3-methoxyphenyl)tetrahydro-pyran-4-carbonitrile (4.1 g, 28%th).1H NMR (400MHz, CDCl3) δ7.34 (t, 1 H), 7.07 (ddd, 1 H), 7.03 (t, 1 H), 6.88 (ddd, 1 H), 4.11-4.04 (m, 2H), 3.90 (td, 2H), 3.83 (s, 3H), 2.18-2.00 (m, 4H).
References:
EP1593679,2005,A1 Location in patent:Page/Page column 25-26

5414-19-7
202 suppliers
$6.00/5g

19924-43-7
269 suppliers
$9.00/10g

179420-73-6
14 suppliers
$323.00/1g