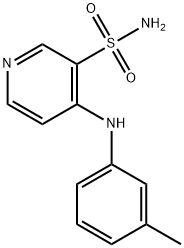
4-(3'-Methylphenyl)amino-3-pyridinesulfonamide synthesis
- Product Name:4-(3'-Methylphenyl)amino-3-pyridinesulfonamide
- CAS Number:72811-73-5
- Molecular formula:C12H13N3O2S
- Molecular Weight:263.32
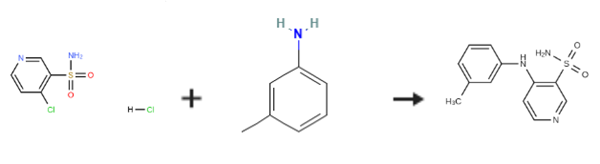
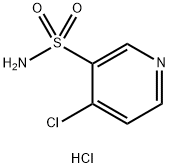
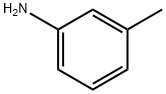
EXAMPLE 1; Preparation of 4-(3-methylphenyl)aminopyridine-sulfonamide; 2L three-neck flask, equipped with a mechanical stirrer, thermometer and condenser, was charged with water (500 ml) and 4-chloro-3- pyridinesulfonamide hydrochloride (100g, 0.44 mol). To this suspension was added m-toluidine (49.2 ml, 0.46 mol) at room temperature. The reaction mixture was heated to 90°C for a minimum period of 3 h. The progress of the reaction was followed by HPLC. After completion, the mixture was cooled to room temperature. The pH of the reaction was then adjusted carefully to pH 7-8 with sat. NaHC03 (ca. 1.1 l). The product was precipitated out and isolated by vacuum filtration as beige solid (126.2 g wet weight). The product was then dissolved in MeOH (1.0 l) at room temperature and charged with Darco KB (25g). The solution was refluxed for 0.5 h and then filtered through a patch of celite to remove Darco KB, while still hot, and rinsed with hot MeOH (200 ML). The filtrate was then charged with water (1.2 l) and stirred for a minimum of 1 h at room temperature. The product, which had precipitated out, was isolated by vacuum filtration to obtain a solid 106.3 g (92percent wet weight =>99.8percent purity a/a). 1H NMR (d6-DMSO) ; 2.30 (s, 3H), 7.00-7. 15 (m. 5H), 7.32 (m, 1H), 7.75 (brs, 1.5H), 8.05 (brs, 0.5H), 8.25 (d, 1H), 8.68 (s, 1H).
777854-85-0
13 suppliers
inquiry
108-44-1
385 suppliers
$9.00/1g

72811-73-5
293 suppliers
$49.00/25mg
Yield:72811-73-5 92%
Reaction Conditions:
Stage #1:4-chloro-3-pyridinesulfonamide hydrochloride;1-amino-3-methylbenzene in water at 90; for 3 h;
Stage #2: with sodium hydrogencarbonate in water; pH=7 - 8 at 20;
Steps:
1 EXAMPLE 1; Preparation of 4-(3-methylphenyl)aminopyridine-sulfonamide
EXAMPLE 1; Preparation of 4-(3-methylphenyl)aminopyridine-sulfonamide; 2L three-neck flask, equipped with a mechanical stirrer, thermometer and condenser, was charged with water (500 ml) and 4-chloro-3- pyridinesulfonamide hydrochloride (100g, 0.44 mol). To this suspension was added m-toluidine (49.2 ml, 0.46 mol) at room temperature. The reaction mixture was heated to 90°C for a minimum period of 3 h. The progress of the reaction was followed by HPLC. After completion, the mixture was cooled to room temperature. The pH of the reaction was then adjusted carefully to pH 7-8 with sat. NaHC03 (ca. 1.1 l). The product was precipitated out and isolated by vacuum filtration as beige solid (126.2 g wet weight). The product was then dissolved in MeOH (1.0 l) at room temperature and charged with Darco KB (25g). The solution was refluxed for 0.5 h and then filtered through a patch of celite to remove Darco KB, while still hot, and rinsed with hot MeOH (200 ML). The filtrate was then charged with water (1.2 l) and stirred for a minimum of 1 h at room temperature. The product, which had precipitated out, was isolated by vacuum filtration to obtain a solid 106.3 g (92% wet weight =>99.8% purity a/a). 1H NMR (d6-DMSO) ; 2.30 (s, 3H), 7.00-7. 15 (m. 5H), 7.32 (m, 1H), 7.75 (brs, 1.5H), 8.05 (brs, 0.5H), 8.25 (d, 1H), 8.68 (s, 1H).
References:
TORCAN CHEMICAL LTD. WO2004/89904, 2004, A2 Location in patent:Page 7
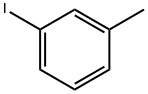
625-95-6
254 suppliers
$14.00/25g
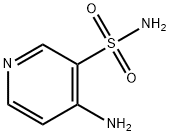
75903-62-7
60 suppliers
$300.00/250 mg

72811-73-5
293 suppliers
$49.00/25mg
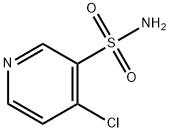
33263-43-3
222 suppliers
$6.00/250mg

108-44-1
385 suppliers
$9.00/1g

72811-73-5
293 suppliers
$49.00/25mg