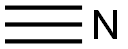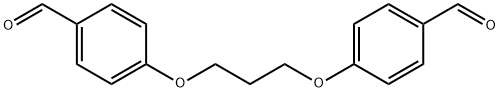
4,4’-(1,3-Propanediyl)dioxydibenzaldhyde synthesis
- Product Name:4,4’-(1,3-Propanediyl)dioxydibenzaldhyde
- CAS Number:3722-80-3
- Molecular formula:C17H16O4
- Molecular Weight:284.31
123-08-0

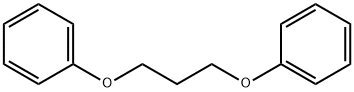
109-64-8

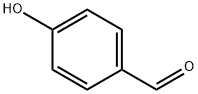
3722-80-3
General procedure: 4-Hydroxybenzaldehyde (1 eq.) is dissolved in acetone. Potassium carbonate (4.5 eq.) dissolved in water was added slowly and the reaction mixture was stirred at room temperature for 10 minutes. Subsequently, 1,3-dibromopropane (0.5 eq.) dissolved in acetone was added dropwise. The reaction mixture was continued to be stirred for 4 hours. The progress of the reaction was monitored by thin layer chromatography (TLC) and after confirming the completion of the reaction, the solvent was removed by evaporation under reduced pressure to give the crude product. The crude product was washed with water, filtered and recrystallized from ethanol-water mixed solvent to give 4,4'-(propane-1,3-diylbis(oxy))dibenzaldehyde (13). Yield: 92%, melting point: 179.6-180.5 °C. 1H-NMR (DMSO-d6, 200 MHz, Me4Si) δ (ppm): 2.21 (t, 2H, -CH2-propylidene-bridged), 4.22 (s, 4H, -O-CH2-), 7.10 (d, J = 8.6 Hz, 4H, aryl proton, H-3, H-5), 7.82 (d, J = 8.6 Hz, 4H, aromatic proton, H-2, H-6), 9.82 (s, 2H, -CHO).13C-NMR (50 MHz, DMSO-d6, Me4Si) δ: 28.7 (C-2'), 85.2 (C-1'), 115.3 (C-3, C-5), 130.1 (C-4), 132.2 (C-2, C-5), 132.2 (C-4), 132.2 (C-4), 132.2 (C-4), 132.2 (C-4), 132.2 (C-4), 132.2 (C-4), 132.2 (C-4) 132.2 (C-2, C-6), 163.8 (C-1), 191.6 (-CHO).MS/FAB+: m/z 285 (M + H+). (calculated value C17H16O4H+ 285.31).

123-08-0
961 suppliers
$5.00/10g

109-64-8
506 suppliers
$10.00/5g

3722-80-3
26 suppliers
$20.00/100mg
Yield:3722-80-3 92%
Reaction Conditions:
Stage #1: 4-hydroxy-benzaldehydewith potassium carbonate in water;acetone at 20; for 0.166667 h;
Stage #2: 1,3-dibromo-propane in water;acetone at 20; for 4 h;
Steps:
General Procedure for the Synthesis of Substituted 1,3-bis(4-carboxaldehydephenoxy)propane (13, 14)
The 4-hydroxybenzaldehyde or vanillin (1 eq) was dissolved in acetone. Potassium carbonate (4.5 eq) dissolved in water was added dropwise and stirred for 10 minutes at room temperature. 1,3-dibromopentane (0.5 eq) dissolved in acetone was added dropwise. Stirring was continued for 4 h. After completion of reaction, determined by thin layer chromatography,the solvent was evaporated under vacuum to afford crude product, which was washed with water, filtered and recrystallized from ethanol-water mixture. 4,4'-[propane-1,3-diylbis(oxy)]dibenzaldehyde (13):Yield: 92%, mp: 179.6 - 180.5 °C. 1H-NMR (DMSO-d6, 200MHz, Me4Si) (ppm): 2.21 (t, 2H, -CH2- propylene bridge),4.22 (s, 4H, -O-CH2-), 7.10 (d, 8.6, 4H, aromatic proton, H-3, H-5), 7.82 (d, 8.6, 4H, aromatic proton H-2, H-6), 9.82 (s,2H -CHO). 13C-RMN (50 MHz, DMSO-d6, Me4Si): 28.7 (C-2’), 85.2 (C-1’), 115.3 (C-3, C-5), 130.1 (C-4), 132.2 (C-2,C-6), 163.8 (C-1), 191.6 (-CHO). MS/FAB+: m/z 285(M+H+). (Calcd for C17H16O4H+ 285.31)
References:
Méndez-Cuesta, Carlos A.;Herrera-Rueda, Miguel ángel;Hidalgo-Figueroa, Sergio;Tlahuext, Hugo;Moo-Puc, Rosa;Chale-Dzul, Juan Bautista;Chan-Bacab, Manuel;Ortega-Morales, Benjamín Otto;Hernández-Nú?ez, Emanuel;Méndez-Lucio, Oscar;Medina-Franco, José L.;NavarreteVázquez, Gabriel [Medicinal Chemistry,2016,vol. 12,p. 1 - 12]

123-08-0
961 suppliers
$5.00/10g

109-64-8
506 suppliers
$10.00/5g
40663-68-1
124 suppliers
$10.00/1g

3722-80-3
26 suppliers
$20.00/100mg
17954-81-3
24 suppliers
$63.61/250mgs:
632-02-0
50 suppliers
inquiry

123-08-0
961 suppliers
$5.00/10g

3722-80-3
26 suppliers
$20.00/100mg

82625-25-0
20 suppliers
$28.60/50MG

123-08-0
961 suppliers
$5.00/10g

142-28-9
281 suppliers
$10.00/1g

3722-80-3
26 suppliers
$20.00/100mg