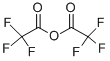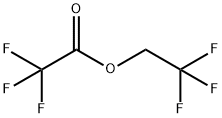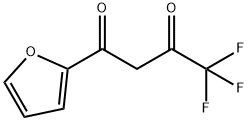
4,4,4-TRIFLUORO-1-(2-FURYL)-1,3-BUTANEDIONE synthesis
- Product Name:4,4,4-TRIFLUORO-1-(2-FURYL)-1,3-BUTANEDIONE
- CAS Number:326-90-9
- Molecular formula:C8H5F3O3
- Molecular Weight:206.12
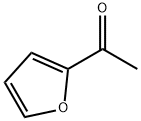
1192-62-7
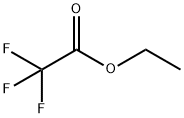
383-63-1

326-90-9
GENERAL STEPS: To a 500 mL three-necked flask were added 20.0 g (181.6 mmol) of 2-acetylfuran, 50 mL of tetrahydrofuran (THF) and 24 mL of ethyl trifluoroacetate. The mixed solution was cooled to 0-3 °C in an ice-water bath, and 1.0 M lithium bis(trimethylsilyl)amide (LiHMDS, 200 mL) solution was slowly added dropwise. After the dropwise addition, the reaction system was allowed to warm up to room temperature naturally and stirred overnight. Upon completion of the reaction, THF was removed by concentration under reduced pressure with a rotary evaporator. the residue was transferred to a partition funnel and extracted with a solution of ethyl acetate and 1.0 M hydrochloric acid. The organic phase was separated, washed with saturated saline, dried over anhydrous sodium sulfate and concentrated again under reduced pressure. The final product was 4,4,4-trifluoro-1-(2-furyl)-1,3-butanedione as a brown semi-solid in a yield of 32.5 g (yield >100%).

1192-62-7
513 suppliers
$5.00/10g

383-63-1
498 suppliers
$10.00/25 g

326-90-9
135 suppliers
$7.00/1g
Yield: 100%
Reaction Conditions:
Stage #1:1-(2-furyl)-1-ethanone;ethyl trifluoroacetate, with lithium hexamethyldisilazane in tetrahydrofuran at 0 - 20;
Stage #2: with hydrogenchloride in water;ethyl acetate
Steps:
1.a
Into a 500 mL flask was weighed 20.0 g (181.6 mmol) of 2-acetylfuran, 50 mL of THF, and 24 mL of ethyl trifluoroacetate. The resulting solution was cooled to 0-3 °C in an ice bath and 1.0 M LiHMDS was added (200 mL). The reaction was allowed to warm to room temperature where it remained overnight. The reaction was then concentrated in vacuo to remove THF and the residue was washed into a separatory funnel with ethyl acetate and 1.0 M HCl. The ethyl acetate was separated, washed with brine, dried (Na2SO4), and concentrated in vacuo. The resulting 4,4,4-trifluoro-1-furan-2-yl-butane-1,3-dione was recovered as a brown semisolid, yield: 32.5 g (100+%).
References:
EXELIXIS, INC. WO2008/73825, 2008, A1 Location in patent:Page/Page column 134

1192-62-7
513 suppliers
$5.00/10g

326-90-9
135 suppliers
$7.00/1g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)