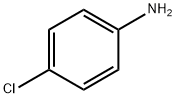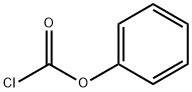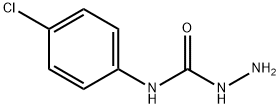
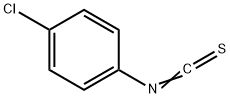
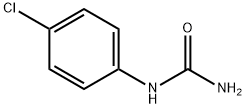
4-(4-CHLOROPHENYL)SEMICARBAZIDE synthesis
- Product Name:4-(4-CHLOROPHENYL)SEMICARBAZIDE
- CAS Number:69194-89-4
- Molecular formula:C7H8ClN3O
- Molecular Weight:185.61
2131-55-7
154 suppliers
$18.00/1g

69194-89-4
15 suppliers
inquiry
Yield:69194-89-4 98%
Reaction Conditions:
with hydrazine hydrate in diethyl ether; for 0.25 h;
Steps:
General synthesis methods of N,3,5-trisubstituted-4,5-dihydro-1H-pyrazole-1-carboxamides (3a-g)
General procedure: The 5 mmol 4-substituted phenylisothiocyanates weredissolved in diethyl ether. In order to stir the mixture,5 mmol hydrazine hydrate was added dropwise and stirredfor 15 min. The obtained 4-substituted phenyl semicarbazides(S1-S4) were filtered, dried, and washed withpetrol ether. 2 mmol chalcone (1a-1i) was dissolved inethanol (10 ml), and then, semicarbazides (S1-S4) wereadded. Finally, solution of 5 mmol NaOH in 1 ml water was added. After the completion of the reaction by TLC,the reaction mixture was poured into ice, and the precipitatewas filtered, dried, and crystallized from ethanol. N-(4-Chlorophenyl)semicarbazide (S1)Yield 98%, mp 282-284 C (EtOH), UV (EtOH), kmax(EtOH) (nm): 204, 247. IR mmax (cm-1): 3330, 3292, 3218,3098, 2980, 2924, 1667, 1592, 1530 1491, 1455, 1405, 1093.
References:
Kocyigit-Kaymakcloglu, Bedia;Beyhan, Nagihan;Tabanca, Nurhayat;Ali, Abbas;Wedge, David E.;Duke, Stephen O.;Bernier, Ulrich R.;Khan, Ikhlas A. [Medicinal Chemistry Research,2015,vol. 24,# 10,p. 3632 - 3644]
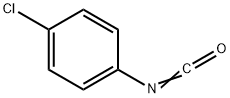
104-12-1
286 suppliers
$10.00/1g

69194-89-4
15 suppliers
inquiry

50882-28-5
10 suppliers
$106.00/250mg

69194-89-4
15 suppliers
inquiry
140-38-5
139 suppliers
$6.00/250mg

69194-89-4
15 suppliers
inquiry