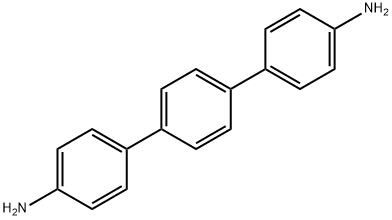
4,4''-DIAMINO-P-TERPHENYL synthesis
- Product Name:4,4''-DIAMINO-P-TERPHENYL
- CAS Number:3365-85-3
- Molecular formula:C18H16N2
- Molecular Weight:260.33
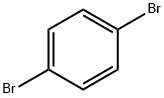
106-37-6
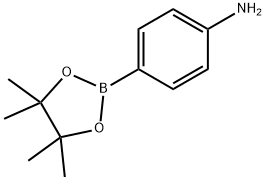
214360-73-3

3365-85-3
1,4-dibromobenzene (0.980 g, 4.15 mmol) and pinacol ester of 4-aminobenzeneboronic acid (2.0 g, 9.13 mmol) were catalyzed by Pd(PPh3)4 (0.3 g, 0.415 mmol) with the addition of K2CO3 (8.6 g, 62.3 mmol), water (10 mL) and toluene (10 mL) in a 50 mL Schlenk flask equipped with a magnetic stirrer. The mixture was rapidly frozen by liquid nitrogen, evacuated to 50 mT, and thawed under static vacuum; this freeze-evacuate-thaw cycle was repeated three times. Subsequently, the flask was backfilled with N2 and a water-cooled condenser, red septum and bubbler were attached to maintain a positive N2 flow under N2 atmosphere. The reaction mixture was heated to 120 °C under N2 protection for 24 h. The progress of the reaction was monitored by TLC. Upon completion of the reaction, it was cooled to room temperature, quenched with water (5 mL) and extracted with EtOAc (3 x 10 mL). The organic phases were combined, washed sequentially with water (3 × 10 mL) and brine (3 × 10 mL), dried over anhydrous Na2SO4 and filtered through diatomaceous earth. The solvent was concentrated under reduced pressure, rotary evaporated at 40 °C, and subsequently purified by silica gel column chromatography (eluent: 35% EtOAc in hexane solution) to afford the white solid product 4,4'-diaminotriphenylene in a yield of 0.560 g (52%). The product was analyzed by 1H NMR (400 MHz, DMSO-d6) δ 7.53 (s, 1H), 7.39-7.35 (d, 1H), 6.66-6.62 (d, 1H), 5.20 (s, 1H); 13C NMR (100 MHz, DMSO) δ 148.18, 137.93, 127.11, 126.82, 125.55, 114.22, 99.51, 39.52; HRMS (ESI-TOF) m/z calculated value C18H16N2 [M+H]+: 261.1393, measured value 261.1355.
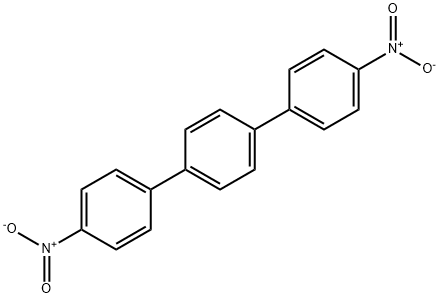
3282-11-9
54 suppliers
$19.00/250mg

3365-85-3
144 suppliers
$9.00/250mg
Yield:3365-85-3 77%
Reaction Conditions:
with 3% Pd/C;hydrazine in ethanolHeating;
Steps:
15
After stirring 3% by weight of a Pd/C catalyst to 1 equivalent of the compound of Formula o-2 in an ethanol solvent, 8 equivalents of an 80% hydrazine solution was slowly added dropwise at room temperature, followed by heating and stirring. After the reaction was completed, a tetrahydrofuran solvent was added, followed by Celite filtration to remove the catalyst. The filtrate was dried with a solvent through a vacuum distillation apparatus. After drying, the solid was filtered using an ethanol solvent to obtain 33.7 g (yield 77%) of a compound of formula o.
References:
LG Chem, Ltd.;Kim Ji-seon;Kang Mi-eun;Kim Min-ju;Song Cheol-jun;Lee Ho-yong KR2020/88054, 2020, A Location in patent:Paragraph 0306-0307; 0312

106-37-6
479 suppliers
$9.00/5g

214360-73-3
338 suppliers
$8.00/1g

3365-85-3
144 suppliers
$9.00/250mg
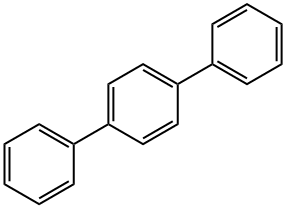
92-94-4
355 suppliers
$16.80/5G

3365-85-3
144 suppliers
$9.00/250mg
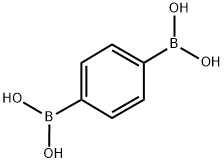
4612-26-4
305 suppliers
$6.00/1g

3365-85-3
144 suppliers
$9.00/250mg
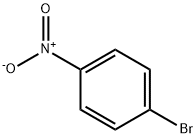
586-78-7
19 suppliers
$14.00/5g

3365-85-3
144 suppliers
$9.00/250mg