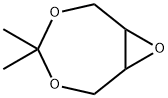
4,4-Dimethyl-3,5,8-trioxabic-yclo[5,1,0]Octane synthesis
- Product Name:4,4-Dimethyl-3,5,8-trioxabic-yclo[5,1,0]Octane
- CAS Number:57280-22-5
- Molecular formula:C7H12O3
- Molecular Weight:144.17
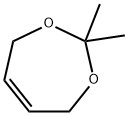
1003-83-4
68 suppliers
$6.00/1g
![4,4-Dimethyl-3,5,8-trioxabic-yclo[5,1,0]Octane](/CAS/GIF/57280-22-5.gif)
57280-22-5
259 suppliers
$8.00/1g
Yield:57280-22-5 85.2%
Reaction Conditions:
with disodium hydrogenphosphate;dihydrogen peroxide;sodium hydroxide in methanol;water;acetonitrile; pH=7 - 9.5 at 60 - 80;Large scale;Green chemistry;
Steps:
4.2
1) To the reaction vessel was charged 1810 kg of compound A-3, 1730 kg of acetonitrile, 1480 kg of methanol and 1920 kgOf water, then add 15. lkg of disodium hydrogen phosphate; stirring heating reaction solution;(3) The reactor was charged with 1990 kg of 27% hydrogen peroxide, and 1M aqueous sodium hydroxide solution 1086 kg, temperature control60-80 ° C; the reaction process to control the pH value of 7-9.5; reaction reactor in the reactor for 8 to 9 hours after stirring to room temperature;(5) In the reaction kettle, 2880kg of saturated brine and 6335kg of methylene chloride were added in this order, and the organic phase was stirred and separated into organic phase. 4010kg of sodium sulfite solution was added. The organic phase was stirred and dried, (2) After drying, the organic phase was filtered and the solvent was distilled off at 40 ° C to obtain crude compound of compound A.(3) The crude compound A was distilled under reduced pressure to give the product A (1820 kg, total yield: 85.2%). The purity of the obtained Compound A showed that the purity of the compound A was 99.6% or more.
References:
Mu Yun;Bu Gonggaofamingren CN106967083, 2017, A Location in patent:Paragraph 0070; 0137-0145
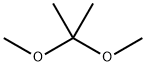
77-76-9
400 suppliers
$9.00/5ml
![4,4-Dimethyl-3,5,8-trioxabic-yclo[5,1,0]Octane](/CAS/GIF/57280-22-5.gif)
57280-22-5
259 suppliers
$8.00/1g