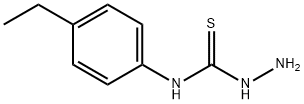
4-(4-ETHYLPHENYL)-3-THIOSEMICARBAZIDE synthesis
- Product Name:4-(4-ETHYLPHENYL)-3-THIOSEMICARBAZIDE
- CAS Number:93693-01-7
- Molecular formula:C9H13N3S
- Molecular Weight:195.28
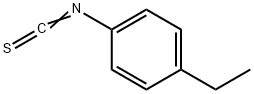
18856-63-8
65 suppliers
$36.00/1g

93693-01-7
19 suppliers
$18.39/1g
Yield:93693-01-7 88%
Reaction Conditions:
with hydrazine hydrate in ethanol;Cooling with ice;
Steps:
4.1.1 The synthesis of 4-(substituted phenyl) thiosemicarbazides (2a-n)
General procedure: To a solution of hydrazine hydrate (5 mmol) in ethanol (10 mL), a suspension of (substituted phenyl)isothiocyanates 1a-n (5 mmol) in ethanol (10 mL) was added dropwise with vigorous stirring and cooling in an ice bath. The mixture was allowed to stand overnight. The obtained solid molecules were filtered, washed three times with water (30 mL), dried, recrystallized from ethanol. The structures of 2a-n in the literature were confirmed by analytical and spectral data by researcher except 2e. 4.1.2 4-(4-Ethylphenyl)thiosemicarbazide (2e) White powder (88%): mp 133-135°C; IR (KBr): υ 3340, 3277, 3190 (NH), 1278 (C-N), 1207 (C=S). 1H NMR (DMSO-d6/ 300MHz) ppm: 0.97 (3H, t, J: 7.6Hz, CH2CH3), 2.37 (2H, q, J: 7.6Hz, CH2CH3), 4.56 (2H, s, NH2), 6.92-6.98 (2H, m, AA′BB′ system phenyl C3,5-H), 7.21-7.31 (2H, m, AA′BB′ system, phenyl C2,6-H), 8.85 (1H, s, N4-H), 9.40 (1H, s, N2-H). 13C NMR (DECOUPLED DMSO-d6/ 75MHz): 16.00 (CH3), 27.89 (CH2), 124.03 (phenyl C2,6), 127.58 (phenyl C3,5), 137.07 (phenyl C1), 139.93 (phenyl C4), 179.63 (C=S).
References:
?zbil, Mehmet;Duran, Gizem Nur;Karal?, Nilgün;Sevin?li, Zekiye ?eyma [Bioorganic Chemistry,2020,vol. 104,art. no. 104202]
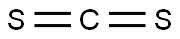
75-15-0
270 suppliers
$40.00/100g
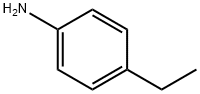
589-16-2
227 suppliers
$10.00/1g

93693-01-7
19 suppliers
$18.39/1g
![Hydrazinecarboxylic acid, 2-[[(4-ethylphenyl)amino]thioxomethyl]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/437736-37-3.gif)
437736-37-3
0 suppliers
inquiry

93693-01-7
19 suppliers
$18.39/1g