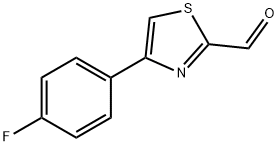
4-(4-FLUOROPHENYL)-1,3-THIAZOLE-2-CARBALDEHYDE synthesis
- Product Name:4-(4-FLUOROPHENYL)-1,3-THIAZOLE-2-CARBALDEHYDE
- CAS Number:383142-69-6
- Molecular formula:C10H6FNOS
- Molecular Weight:207.22
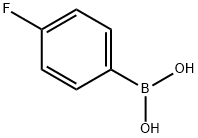
1765-93-1
550 suppliers
$9.00/1g
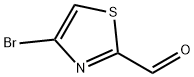
167366-05-4
156 suppliers
$10.00/250mg

383142-69-6
20 suppliers
$70.00/25mg
Yield:383142-69-6 32.9%
Reaction Conditions:
Stage #1: 4-fluoroboronic acid;4-bromo-1,3-thiazole-2-carbaldehydewith caesium carbonate in 1,4-dioxane;water at 20; for 0.0833333 h;Suzuki Coupling;
Stage #2: with tetrakis(triphenylphosphine) palladium(0) in 1,4-dioxane;water;Inert atmosphere;Reflux;
Steps:
1. Suzuki cross-coupling for the preparation of 4a-h, 7a-j and 10a-i
General procedure: Bromoarylaldehyde (1 mmol), aryl or alkenyl-boronicacid (1.2 mmol), and Cs2CO3(2.5 mmol) were dissolved orsuspended in a mixture of 1,4-dioxane (10 mL) and water (5 mL). The resulting mixture was stirred at RT for 5min. Tetrakis (triphenylphosphine) palladium(0) (0.05mmol) was added and the mixture was refluxed for 4-6 h under N2 protection. After cooling to RT, the mixture was dilutedwith CH2Cl2 (10 mL) and the separated aqueous layer wasextracted with CH2Cl2 (3 × 10 mL). The combined organic layers were dried over Na2SO4,filtered, and the solution was concentrated in vacuo to obtain a residue, whichwas purified by silica gel CC using ethyl acetate-petroleum ether gradientelution (1:200-1:4, v/v) to afford the aldehydes.
References:
Zhang, Yang;Zhang, Zhuowei;Wang, Bo;Liu, Ling;Che, Yongsheng [Bioorganic and Medicinal Chemistry Letters,2016,vol. 26,# 8,p. 1885 - 1888] Location in patent:supporting information