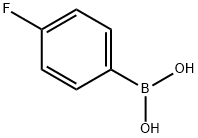
4-Fluorobenzeneboronic acid synthesis
- Product Name:4-Fluorobenzeneboronic acid
- CAS Number:1765-93-1
- Molecular formula:C6H6BFO2
- Molecular Weight:139.92

121-43-7
382 suppliers
$13.00/25mL
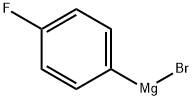
352-13-6
197 suppliers
$40.00/50 mL

1765-93-1
550 suppliers
$9.00/1g
Yield:1765-93-1 92%
Reaction Conditions:
in 2-methyltetrahydrofuran at -5;Inert atmosphere;Temperature;Solvent;Concentration;
Steps:
1.1; 1.2; 1.3; 1.4
(1) replacing the air with nitrogen in each module of the microchannel reactor system through which the material flows, including the pre-incubation module, the first reaction module, the second reaction module and the quenching module; (2) 4-Fluorophenyl magnesium bromide was diluted with 2-methyltetrahydrofuran to a molar concentration of 0.3 mol / L,As the material 1; trimethyl borate with 2-methyl tetrahydrofuran diluted to 0.5mol / L homogeneous mixed solution, as the material 2;The material 1 and the material 2 are respectively introduced into the first pre-heat preservation module 11 and the second pre-heat preservation module 12 shown in Fig. 1 and pre-insulated so as to be -5 ; (3) Both the material 1 and the material 2 are preincreated and passed into the first reaction module. As shown in Fig. 1, the material 1 and the material 2 are mixed and reacted in the first reaction module 21, and then the two reaction modules 22 , The reaction temperature in the first reaction module and the two second reaction modules is -5 , and the total residence time of the reaction liquid in the first reaction module and the two second reaction modules is 22 seconds, Since the concentration of materials 1 and 2 is constant and the influent pipe thickness of the two materials in the first reaction module is the same,It is possible to control the number of moles of 4-fluorophenyl magnesium bromide and trimethyl borate introduced into the first reaction module per unit time by controlling the inflow rate of the materials 1 and 2 in the first reaction module,So that the molar ratio of 4-fluorophenyl magnesium bromide and trimethyl borate to the first reaction module per unit time was 1: 2.0, and the flow rate of the controlled material 1 in this example was 30 ml / min;The flow rate of the control material 2 is 36ml / min. As shown in Fig. 1, the reaction liquid flowing out from the last second reaction module enters the quenching module 31, quenching the reaction liquid by passing 10% dilute hydrochloric acid into the quenching module , 10% hydrochloric acid flow rate of 15ml / min; (4) When the reactor in the various strands of material to a steady state, the collection of quenching reaction by quenching reaction to access 25min of material 1 (750ml) corresponding to the reaction solution as an example,The organic phase was dried and the ethyl acetate was removed by rotary evaporation to obtain 31.05 g of crude 4-fluorobenzeneboronic acid,After adding 300 ml of methylene chloride at 20 ° C, 28.96 g of white crystalline powder of 4-fluorobenzene boronic acid was obtained by filtration and drying, and the molar yield was 92.0%.
References:
Heilongjiang Xinchang Biotechnology Development Co., Ltd;Yang, Kun;An, Yongsheng CN105753835, 2016, A Location in patent:Paragraph 0041; 0042; 0043; 0044; 0045
460-00-4
636 suppliers
$6.00/10g

1765-93-1
550 suppliers
$9.00/1g

121-43-7
382 suppliers
$13.00/25mL

460-00-4
636 suppliers
$6.00/10g

1765-93-1
550 suppliers
$9.00/1g
352-34-1
380 suppliers
$7.00/5g

1765-93-1
550 suppliers
$9.00/1g
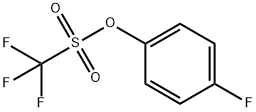
132993-23-8
23 suppliers
inquiry

1765-93-1
550 suppliers
$9.00/1g