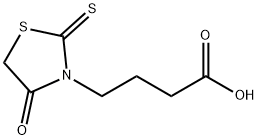
4-(4-OXO-2-THIOXO-THIAZOLIDIN-3-YL)-BUTYRIC ACID synthesis
- Product Name:4-(4-OXO-2-THIOXO-THIAZOLIDIN-3-YL)-BUTYRIC ACID
- CAS Number:18623-60-4
- Molecular formula:C7H9NO3S2
- Molecular Weight:219.28
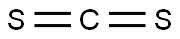
75-15-0
272 suppliers
$40.00/100 g
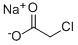
3926-62-3
299 suppliers
$16.69/250g
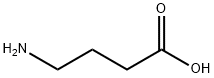
56-12-2
920 suppliers
$5.00/100mg

18623-60-4
42 suppliers
$65.00/50mg
Yield: 91%
Reaction Conditions:
Stage #1:carbon disulfide;sodium monochloroacetic acid;4-amino-n-butyric acid with potassium hydroxide in water at 20; for 48 h;
Stage #2: with hydrogenchloride in water for 1 h;Reflux;
Steps:
4-(4-Oxo-2-sulfanylidene-1,3-thiazolidin-3-yl)butanoic acid (2)
A flat-bottom flask equipped with a reflux condenser was charged with 50 mmol of γ-aminobutyric acid, 5.7 g (5.5 mmol) of carbon disulfide, 5.6 g (100 mmol) of potassium hydroxide, and 15 mL of water. The mixture was stirred with a magnetic stirrer, and a solution of 5.2 g (5.5 mmol) of chloroacetic acid preliminarily neutralized with 5.5 mmol of sodium hydrogen carbonate in 25 mL of water was added dropwise with stirring over a period of 15 min. The mixture was left to stand at ~20°C for 2 days, 20 mL of 6 N aqueous HCl was added to the resulting solution, and the mixture was refluxed for 1 h. The mixture was cooled, and the precipitate was filtered off and recrystallized twice in succession from dilute acetic acid and ethanol. Yield 91%, mp 121-122°C.
References:
Horishny, V. Ya.;Matiychuk, V. S. [Russian Journal of Organic Chemistry,2020,vol. 56,# 7,p. 1146 - 1152][Zh. Org. Khim.,2020,vol. 56,# 7,p. 1021 - 1029,9]
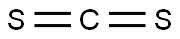
75-15-0
272 suppliers
$40.00/100 g
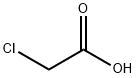
79-11-8
0 suppliers
$24.00/25g

56-12-2
920 suppliers
$5.00/100mg

18623-60-4
42 suppliers
$65.00/50mg