
4-(4-TRIFLUOROMETHYLPHENYL)BENZYLAMINE synthesis
- Product Name:4-(4-TRIFLUOROMETHYLPHENYL)BENZYLAMINE
- CAS Number:356058-18-9
- Molecular formula:C14H12F3N
- Molecular Weight:251.25
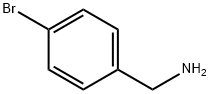
3959-07-7
206 suppliers
$10.00/1g
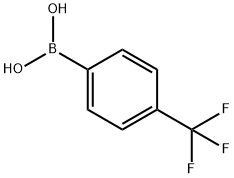
128796-39-4
436 suppliers
$10.00/5g

356058-18-9
13 suppliers
inquiry
Yield:356058-18-9 85%
Reaction Conditions:
with (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride;potassium carbonate in 2-methyltetrahydrofuran;water at 80; for 15 h;Inert atmosphere;
Steps:
147.A Step A: Preparation of (4′-(Trifluoromethyl)[1,1′-biphenyl]-4-yl)methanamine
Step A:
Preparation of (4'-(Trifluoromethyl)[1,1'-biphenyl]-4-yl)methanamine
To a nitrogen purged flask containing 4-trifluoromethylphenyl boronic acid (76.6 g, 403 mmol), [1,1'-bis(di-tert-butylphosphino)ferrocene]dichloropalladium(II) (6.07 g, 9.31 mmol), and potassium carbonate (83.6 g, 605 mmol) were added 4-bromobenzylamine (75.0 g, 403 mmol), N2 sparged 2-methyltetrahydrofuran (750 mL) and N2 sparged water (750 mL).
The mixture was then warmed to 80° Celsius for 15 h.
The mixture was cooled to RT, the layers were separated and the organic layer was washed with brine, dried over magnesium sulfate and filtered through celite.
The filtrate was concentrated to dryness to afford the title compound (114.37 g, 85% purity, 96%). MS (ESI): mass calcd. for C14H12F3N, 251.10; m/z found, 252.1 [M+H]+. 1H NMR (400 MHz, CDCl3) δ 7.68 (s, 4H), 7.58 (d, J=7.9, 2H), 7.42 (d, J=7.9, 2H) 3.94 (s, 2H).
Absolute stereochemistry was determined by alternative synthesis starting with the product of Example 2.
References:
US2014/275029,2014,A1 Location in patent:Paragraph 0582
![4'-(TRIFLUOROMETHYL)[1,1'-BIPHENYL]-4-CARBONITRILE](/StructureFile/ChemBookStructure8/GIF/CB3119132.gif)
140483-60-9
19 suppliers
inquiry

356058-18-9
13 suppliers
inquiry