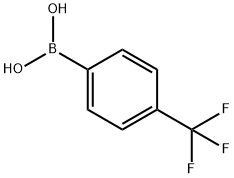
4-Trifluoromethylphenylboronic acid synthesis
- Product Name:4-Trifluoromethylphenylboronic acid
- CAS Number:128796-39-4
- Molecular formula:C7H6BF3O2
- Molecular Weight:189.93

121-43-7
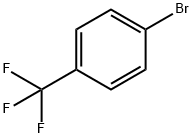
402-43-7

128796-39-4
[Example 4] In this embodiment, the synthesis of 9-[4-(carbazol-9-yl)phenyl]-10-(4-trifluoromethylphenyl)anthracene (CF3CzPA) represented by structural formula (42) is described. [Step 1] Synthesis of 9-bromo-10-(4-trifluoromethylphenyl)anthracene (i) Synthesis of 4-(trifluoromethyl)phenylboronic acid The synthesis scheme of 4-(trifluoromethylphenylboronic acid) is shown in (E-1). (1) 33 g (0.15 mol) of p-bromobenzotrifluoride was added to a 500 mL three-necked flask under nitrogen protection. Subsequently, 200 mL of tetrahydrofuran (THF) was added and the mixture was stirred. The mixed solution was cooled to -78 °C and 100 mL (0.16 mol) of n-butyllithium (1.6 mol/L) solution was slowly added dropwise through a dropping funnel. After the dropwise addition was completed, stirring was continued for 1 hour by keeping it at -78°C. Then, 22.3 mL (0.20 mol) of trimethyl borate was added and the reaction mixture was gradually warmed to room temperature and stirred for about 12 hours. After the reaction was completed, 100 mL of dilute hydrochloric acid (1 mol/L) was added to the reaction solution and stirring was continued for 1 hour. The aqueous layer was extracted three times with ethyl acetate, the organic layers were combined, washed once with saturated brine and dried with magnesium sulfate. The magnesium sulfate was removed by filtration and the filtrate was concentrated to obtain the solid product. The solid was washed with chloroform to give a final 15 g of white solid target product 4-trifluoromethylphenylboronic acid in 54% yield.

67-56-1
790 suppliers
$7.29/5ml-f
131765-96-3
31 suppliers
$42.79/1g

402-43-7
445 suppliers
$6.00/10g

128796-39-4
436 suppliers
$10.00/5g
Yield:128796-39-4 90%
Reaction Conditions:
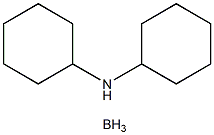
Stage #1:dicyclohexylamine borane complex;p-trifluoromethylphenyl bromide with magnesium in tetrahydrofuran at 70;
Stage #2:methanol in tetrahydrofuran at 0; for 1 h;
Stage #3: with hydrogenchloride;water in methanol at 20; for 1 h;
Steps:
Addition under Barbier conditions (procedure A)
General procedure: A solution in THF (4 mL) of DIPAB (863 mg, 7.5 mmol), Mg (182 mg, 7.5 mmol) and arylbromide (5 mmol) was stirred at 70 °C until no starting arylbromide remains (TLC). The reaction mixture was cooled down to 0 °C and quenched slowly with 7 mL of MeOH. After 1h, volatile were removed under reduced pressure and the resulting solid was dissolved in 1N HCl/MeOH (7/3). After 1h at room temperature, 100 mL of AcOEt were added, the organic phase was washed with 1N HCl (30 mL) and brine (3×30 mL). Organic phases were concentrated under reduced pressure yielding a solid which was recrystallized from H2O.
References:
Marciasini, Ludovic D.;Richard, Jimmy;Cacciuttolo, Bastien;Sartori, Guillaume;Birepinte, Melodie;Chabaud, Laurent;Pinet, Sandra;Pucheault, Mathieu [Tetrahedron,2019,vol. 75,# 2,p. 164 - 171]

121-43-7
381 suppliers
$13.00/25mL

402-43-7
445 suppliers
$6.00/10g

128796-39-4
436 suppliers
$10.00/5g

402-43-7
445 suppliers
$6.00/10g

128796-39-4
436 suppliers
$10.00/5g
455-14-1
522 suppliers
$5.00/10g

128796-39-4
436 suppliers
$10.00/5g

67-56-1
790 suppliers
$7.29/5ml-f
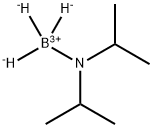
55124-35-1
25 suppliers
$60.00/100mg

402-43-7
445 suppliers
$6.00/10g

128796-39-4
436 suppliers
$10.00/5g