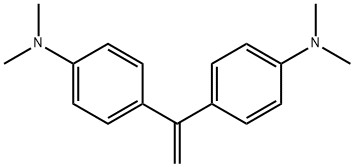
4,4'-VINYLIDENEBIS(N,N-DIMETHYLANILINE) synthesis
- Product Name:4,4'-VINYLIDENEBIS(N,N-DIMETHYLANILINE)
- CAS Number:7478-69-5
- Molecular formula:C18H22N2
- Molecular Weight:266.38
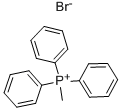
1779-49-3
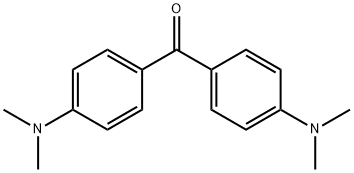
90-94-8

7478-69-5
The synthesis of BDADPE was carried out by the classical Wittig reaction under argon atmosphere using 4,4'-bis(dimethylamino)benzophenone as substrate (for details, refer to the method of Hirao [29]). The procedure was as follows: methyltriphenylphosphonium bromide (45.58 g, 127.6 mmol) was added to a round-bottomed flask equipped with a reflux condenser and a magnetic stirrer with freshly distilled tetrahydrofuran (THF, 500 mL). Subsequently, potassium tert-butoxide (138.4 mL of a 1.0 M THF solution, 138.4 mmol) was slowly added dropwise to the reaction flask through a constant pressure dropping funnel. The reaction mixture was stirred at 0 °C for 2 hours. Next, a solution of anhydrous THF (690 mL) of 4,4'-bis(dimethylamino)benzophenone (21.4 g, 79.75 mmol) was added dropwise to the reaction mixture at 0 °C via a constant pressure dropping funnel. The orange-brown reaction mixture was heated and refluxed with stirring for 7 h. The reaction was quenched with distilled water upon completion. The resulting mixture was extracted three times with ether. The combined ether layers were washed sequentially with aqueous NaHCO3 and saturated aqueous NaCl and then dried with anhydrous MgSO4. After filtration, the ether layer was poured into hexane to precipitate triphenylphosphine oxide. Purification by fast column chromatography (eluent: petroleum ether/ethyl acetate, 2:1 v/v) afforded BDADPE in 92% yield (19.52 g, 73.37 mmol) with an Rf-value of 0.76. The structure of the product was confirmed by 1H NMR (CDCl3, 400 MHz): δ 2.96 (s, 12H, 2×N(CH3)2), 5.19 (s , 2H, CH2=C), 6.68-6.70 (d, 4H, aromatic proton neighbor of N(CH3)2), 7.25-7.28 (d, 4H, aromatic proton with N(CH3)2).

1779-49-3
623 suppliers
$5.00/10g

90-94-8
226 suppliers
$29.00/100g

7478-69-5
42 suppliers
$60.00/100mg
Yield: 92%
Reaction Conditions:
Stage #1:Methyltriphenylphosphonium bromide with potassium tert-butylate in tetrahydrofuran at 0; for 2 h;Inert atmosphere;
Stage #2:bis(p-dimethylaminophenyl)methanone in tetrahydrofuran at 0;Reflux;Wittig Olefination;
Steps:
2.3 Synthesis of 1,1-bis(4-dimethylaminophenyl)ethylene
The synthesis of BDADPE was conducted by the classic Wittig reaction using 4,4′-bis(dimethylamino)benzophenone as substrate under an argon atmosphere (described in detail by Hirao [29]). Typically, methyltriphenylphosphonium bromide (45.58g, 127.6mmol) and freshly distilled THF (500mL) were added into a round bottom flask equipped with a reflux condenser and a magnetic bar. Potassium tert-butoxide (138.4mL of a 1.0M solution in THF, 138.4mmol) was then added dropwise to the reaction flask via constant pressure funnel. The reaction mixture was stirred for 2h at 0°C. Then the solution of 4,4′-bis(dimethylamino)benzophenone (21.4g, 79.75mmol) in dry THF (690mL) was dropped to the reaction mixture via constant pressure funnel at 0°C. The orange-brown reaction mixture was heated to reflux for 7h with stirring and then quenched with distilled water. The resultant mixture was extracted with ether three times. Such ethereal layer was washed with aqueous NaHCO3 solution and saturated aqueous NaCl solution and then dried over MgSO4. After filtration, the ethereal layer was poured into hexane to precipitate triphenylphosphine oxide. Flash column chromatography (petroleum ether/ethyl acetate 2:1 v/v) gave BDADPE in 92% yield (19.52g, 73.37mmol) and Rf=0.76. 1H NMR (CDCl3, 400MHz): δ 2.96 (s, 12H, 2×N(CH3)2), 5.19 (s, 2H, CH2=C), 6.68-6.70 (d, 4H, aromatic proton ortho to N(CH3)2), 7.25-7.28 (d, 4H, aromatic proton meta to N(CH3)2).
References:
Wu, Lingling;Wang, Yanshai;Wang, Yurong;Shen, Kaihua;Li, Yang [Polymer,2013,vol. 54,# 12,p. 2958 - 2965]
78-08-0
281 suppliers
$11.19/100G
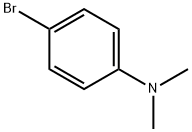
586-77-6
438 suppliers
$5.00/10g

7478-69-5
42 suppliers
$60.00/100mg
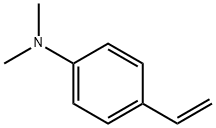
2039-80-7
76 suppliers
$32.00/100mg
![N-(4-{2-[4-(dimethylamino)phenyl]vinyl}phenyl)-N,N-dimethylamine](/CAS/20180601/GIF/22210-79-3.gif)
22210-79-3
1 suppliers
inquiry

78-08-0
281 suppliers
$11.19/100G

586-77-6
438 suppliers
$5.00/10g

7478-69-5
42 suppliers
$60.00/100mg
![N-(4-{2-[4-(dimethylamino)phenyl]vinyl}phenyl)-N,N-dimethylamine](/CAS/20180601/GIF/22210-79-3.gif)
22210-79-3
1 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

7478-69-5
42 suppliers
$60.00/100mg

90-94-8
226 suppliers
$29.00/100g

7478-69-5
42 suppliers
$60.00/100mg