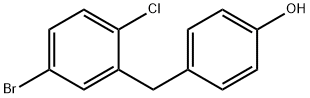
4-(5-broMo-2-chlorobenzyl)phenol synthesis
- Product Name:4-(5-broMo-2-chlorobenzyl)phenol
- CAS Number:864070-18-8
- Molecular formula:C13H10BrClO
- Molecular Weight:297.57
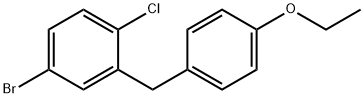
461432-23-5

864070-18-8
Example 1 Synthesis of 4-(5-bromo-2-chlorobenzyl)phenol: To a stirred dichloromethane solution (250 mL) of 4-bromo-1-chloro-2-(4-ethoxybenzyl)benzene (8.47 g, 0.026 mol) was added slowly and dropwise to a dichloromethane solution (8 mL, 4M) of tribromoborane at -78 °C. The reaction mixture was continued to be stirred at -78°C for 30 minutes, then brought to room temperature and stirred for 1 hour. Upon completion of the reaction, the reaction was quenched by slow addition of saturated aqueous sodium bicarbonate solution (200 mL) and the mixture was extracted three times with ethyl acetate. The organic phases were combined, washed once with saturated aqueous sodium chloride solution and dried over anhydrous sodium sulfate. The solvent was removed by concentration under reduced pressure and the residue was purified by silica gel column chromatography (eluent: petroleum ether/ethyl acetate, 10:1) to afford the target product 4-(5-bromo-2-chlorobenzyl)phenol. Yield: 6.85 g, 89%. LC-MS (ESI): m/z = 297/299 (Cl)[M + H]+.

461432-23-5
515 suppliers
$30.00/1g

864070-18-8
185 suppliers
$35.00/1g
Yield:864070-18-8 98%
Reaction Conditions:
Stage #1:4-bromo-1-chloro-2-[(4-ethoxyphenyl)methyl]benzene with boron trichloride in dichloromethane at 0; for 0.5 h;Inert atmosphere;
Stage #2: with boron tribromide in dichloromethane at 0;
Steps:
To a solution of 4-bromo-1-chloro-2-(4-ethoxy-benzyl)-benzene (10.0 g, 30.71 mmol) in dichloromethane (40.0 mL) cooled to 0 degrees Celsius under nitrogen was added drop- wise over 30 minutes a 1 M solution of boron trichloride in dichloromethane (34 mL, 34.0 mmol). After the addition was complete, the reaction was allowed to warm to room temperature overnight (-16 hours). The reaction mixture was cooled to 0 degrees Celsius and an aqueous solution of 1 N hydrochloric acid was added. The resulting mixture was stirred for 30 minutes and then extracted using dichloromethane. The organic layer was separated and the aqueous layer was extracted two times with dichloromethane. The combined organic layers were dried over magnesium sulfate, filtered and concentrated under reduced pressure. The TLC showed only 50% conversion. The crude material was redissolved in dichloromethane (40 mL), cooled to 0 degrees Celsius, and a 1M solution of boron tribromide in dichloromethane (31 mL, 31 mmol) was added dropwise. The reaction mixture was allowed to warm to room temperature over the weekend (-55 hours). The reaction mixture was cooled to 0 degrees Celsius and an aqueous solution of 1 N hydrochloric acid was added dropwise. The resulting mixture was stirred for 30 minutes and then extracted usingdichloromethane. The organic layer was separated and the aqueous layer was extracted two times with dichloromethane. The combined organic layers were dried over magnesium sulfate, filtered and concentrated under reduced pressure. The reaction was purified by flash chromatography over silica gel using the ISCO automated chromatography unit (120 g silica gel column) and eluting with a gradient of 0 to 100% ethyl acetate in heptane. 9 g (98% yield) of the desired product obtained as a white solid.
References:
PFIZER INC.;MASCITTI, Vincent WO2011/51864, 2011, A1 Location in patent:Page/Page column 27-28
1360568-68-8
40 suppliers
inquiry

864070-18-8
185 suppliers
$35.00/1g
21739-92-4
694 suppliers
$8.00/10g

864070-18-8
185 suppliers
$35.00/1g

21900-52-7
86 suppliers
$12.00/250mg

864070-18-8
185 suppliers
$35.00/1g
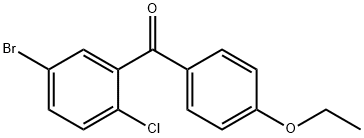
461432-22-4
352 suppliers
$5.00/250mg

864070-18-8
185 suppliers
$35.00/1g