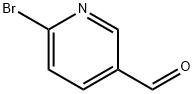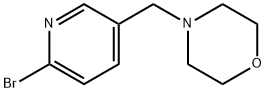
4-[(6-bromopyridin-3-yl)methyl]morpholine synthesis
- Product Name:4-[(6-bromopyridin-3-yl)methyl]morpholine
- CAS Number:364793-93-1
- Molecular formula:C10H13BrN2O
- Molecular Weight:257.13
Yield:364793-93-1 85%
Reaction Conditions:
Stage #1: morpholine;6-bromonicotinaldehydewith sodium tris(acetoxy)borohydride;acetic acid in 1,2-dichloro-ethane at 20; for 0.666667 h;
Stage #2: with sodium hydrogencarbonate in 1,2-dichloro-ethane;
Steps:
To a RB flask containing formylpyridine (9.347 g, 1 equiv.) in 175 mL 1,2-dichloroethane (3.5 mL/mmol) was added morpholine (4.7 mL, 1.07 equiv.) followed by NaBH(OAc)3 (14.819 g, 1.4 equiv.) and acetic acid (3.1 mL, 1.07 equiv.). The flask was loosely capped and the mixture was stirred at r.t. Mixture gets slightly warm. After 40 min, the reaction was quenched with saturated NaHCO3. When the gas evolution was greatly reduced, 1M NaOH was added to bring the pH to 8-9. The two layers were separated and the aqueous layer was extracted with DCM (×3). The organic layer was dried over Na2SO4, filtered and solvent was removed in vacuo. The product was filtered through silica with 1000 mL 100:1 EtOAc:NH4OH to remove baseline material. The material was dissolved in 15 mL EtOAc and 150 mL hexanes was added. The mixture was allowed to sit overnight at 4° C. to crystallize. The supernatant was decanted off and the crystals were washed with a little hexanes which was decanted off. The crystals were transferred to another flask using DCM. LC-MS showed only product. The solvent from the supernatant was removed in vacuo. The remaining mixture was purified by flash column (6.5×8.5 cm silica) using 500 mL 8:2 EtOAc; 1400 mL 100:1 EtOAc:NH4OH. All product fractions were combined giving 10.931 g (85%) of the 4-((6-bromopyridin-3-yl)methyl)morpholine as a yellow solid.In a RB flask, a mixture of 4-((6-bromopyridin-3-yl)methyl)morpholine (10.959 g, 1 equiv.), 4-methyl-3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)aniline (10.478 g, 1.05 equiv.), 2M potassium phosphate solution (42.5 mL, 2 equiv.) and Pd(PPh3)4 (2.479, 0.050 equiv.)) in 210 mL dioxane (5 mL/mmol) was sparged with argon for 5 min. The flask was fitted with a septum and argon balloon and the mixture was stirred at 100° C. (amber solution). After 27 h, the mixture was allowed to cool then volume was reduced by at least half in vacuo. The remainder was diluted with water and extracted with EtOAc (×3). The organic layers were washed with brine, dried over Na2SO4, filtered and solvent was removed in vacuo. The material was purified by column chromatography on silica eluting with DCM:MeOH to give 10.268 g (85%) of 4-methyl-3-(5-(morpholinomethyl)pyridin-2-yl)aniline as a very viscous dark amber oil.
References:
US2010/41642,2010,A1 Location in patent:Page/Page column 20

110-91-8
710 suppliers
$9.00/1g
101990-45-8
109 suppliers
$44.00/100mg
![4-[(6-bromopyridin-3-yl)methyl]morpholine](/CAS/GIF/364793-93-1.gif)
364793-93-1
45 suppliers
inquiry