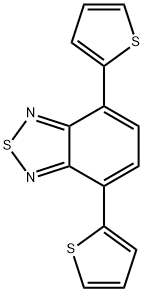
4,7-Bis(thiophen-2-yl)benzo[c][1,2,5]thiadiazole synthesis
- Product Name:4,7-Bis(thiophen-2-yl)benzo[c][1,2,5]thiadiazole
- CAS Number:165190-76-1
- Molecular formula:C14H8N2S3
- Molecular Weight:300.42
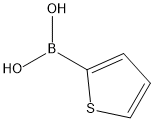
6165-68-0
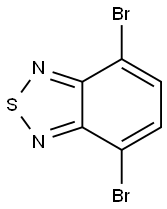
15155-41-6
![4,7-Bis(thiophen-2-yl)benzo[c][1,2,5]thiadiazole](/CAS/20150408/GIF/165190-76-1.gif)
165190-76-1
General procedure for the synthesis of 4,7-bis(thiophen-2-yl)benzo[c][1,2,5]thiadiazoles from 2-thiopheneboronic acid and 4,7-dibromo-2,1,3-benzothiadiazole: To a mixture of 4,7-dibromobenzo[c][1,2,5]thiadiazole (147 mg, 0.50 mmol, 1 equiv.), thiophene-2-ylboronic acid (192 mg, 1.50 mmol, 3 eq.) and K3PO4-H2O (0.90 g, 3.0 mmol, 6 eq.) was added to a mixture of THF (900 μL), followed by a THF stock solution of Pd(PAd3) (100 μL, 0.25 μmol Pd). The reaction mixture was stirred for 1 h at room temperature. Upon completion of the reaction, the reaction mixture was diluted with ethyl acetate and subsequently extracted with water. The organic layers were combined, the solvent was evaporated and the crude product was purified by fast chromatography. After drying, 143 mg (95% yield) of the target compound was obtained as an orange solid.The NMR spectral data were in agreement with literature values.

6165-68-0
392 suppliers
$14.00/5g

15155-41-6
325 suppliers
$20.00/1g
![4,7-Bis(thiophen-2-yl)benzo[c][1,2,5]thiadiazole](/CAS/20150408/GIF/165190-76-1.gif)
165190-76-1
101 suppliers
$40.00/250mg
Yield:165190-76-1 95%
Reaction Conditions:
with O4P(3-)*3K(1+)*5H2O;tris(1-adamantyl)phosphine;{2-[((acetyl-κO)amino)phenyl-κC](tri-1-adamantylphosphine)palladium}(p-toluenesulfonate) in tetrahydrofuran at 20; for 1 h;Suzuki-Miyaura Coupling;
Steps:
To a mixture of 4,7-dibromobenzo[cJ{1,2,5]thiadiazole (147 mg, 0.50 mmol, 1 equiv),thiophen-2-ylboronic acid (192 mg, 1.50 mmol, 3 equiv), and K3P045H20 (0.90 g, 3.0 mmol, 6 equiv) was added THF (900 jiL) then a THF stock solution of 3 and PAd3 (100 iL, 0.25 jtmol ofPd/PAds). The mixture was stirred at room temperature for 1 h. The reaction mixture was diluted with ethyl acetate then extracted with water. The combine organic layers were evaporated andthe crude product was purified by flash chromatography. After drying, 143 mg (95%) of 40 was obtained as an orange solid. NMR spectroscopic data agreed with literature values.
References:
THE TRUSTEES OF PRINCETON UNIVERSITY;CARROW, Brad P.;CHEN, Liye WO2017/75581, 2017, A1 Location in patent:Page/Page column 31
188290-36-0
2 suppliers
inquiry

15155-41-6
325 suppliers
$20.00/1g
![4,7-Bis(thiophen-2-yl)benzo[c][1,2,5]thiadiazole](/CAS/20150408/GIF/165190-76-1.gif)
165190-76-1
101 suppliers
$40.00/250mg

15155-41-6
325 suppliers
$20.00/1g
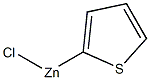
81745-84-8
2 suppliers
inquiry
![4,7-Bis(thiophen-2-yl)benzo[c][1,2,5]thiadiazole](/CAS/20150408/GIF/165190-76-1.gif)
165190-76-1
101 suppliers
$40.00/250mg

15155-41-6
325 suppliers
$20.00/1g
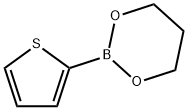
197024-83-2
31 suppliers
$75.00/100mg
![4,7-Bis(thiophen-2-yl)benzo[c][1,2,5]thiadiazole](/CAS/20150408/GIF/165190-76-1.gif)
165190-76-1
101 suppliers
$40.00/250mg

15155-41-6
325 suppliers
$20.00/1g
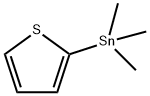
37496-13-2
20 suppliers
inquiry
![4,7-Bis(thiophen-2-yl)benzo[c][1,2,5]thiadiazole](/CAS/20150408/GIF/165190-76-1.gif)
165190-76-1
101 suppliers
$40.00/250mg