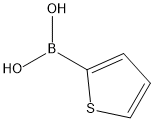
2-Thiopheneboronic acid synthesis
- Product Name:2-Thiopheneboronic acid
- CAS Number:6165-68-0
- Molecular formula:C4H5BO2S
- Molecular Weight:127.96
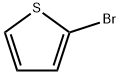
1003-09-4

6165-68-0
General procedure for the synthesis of 2-thiophene boronic acid from 2-bromothiophene: Triphenylphosphine (0.131 g, 0.5 mmol, 20 mol%), p-iodoanisole (0.585 g, 2.5 mmol), and triethylamine (1.78 mL, 12.5 mmol) were added sequentially to a 50 mL round-bottomed flask (equipped with a side arm, condenser, and stirring bar). The reaction system was degassed three times by alternating vacuum and argon displacement. Palladium dichloride (0.023 g, 0.13 mmol, 5 mol%) was added under positive argon pressure. After stirring at room temperature for 15 minutes, diisopropylaminoborane (5 mL, 1 M THF solution, 5 mmol) was added and again degassed three times by alternating vacuum and argon displacement. The reaction mixture was heated to reflux and kept at reflux for 12 hours. Upon completion of the reaction, the mixture was cooled to 0°C and 6 mL of methanol was slowly added (note: this process is exothermic and accompanied by hydrogen release). After stirring for 15 minutes, all solvent was removed by distillation under reduced pressure to give a black solid. The solid was dissolved with 3M sodium hydroxide solution (8 mL) and subsequently washed with hexane (3 x 10 mL). The aqueous layer was cooled to 0°C (ice bath) and acidified with concentrated hydrochloric acid to pH ≤ 1, at which point 2-thiopheneboronic acid precipitated as a white solid. The aqueous layer was extracted with ether (3 x 10 mL), the organic phases were combined, dried with magnesium sulfate and filtered. Finally, the solvent was removed by distillation under reduced pressure to obtain 2-thiopheneboronic acid as a white solid.
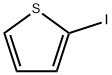
3437-95-4
250 suppliers
$6.00/5g

6165-68-0
391 suppliers
$14.00/5g
Yield:6165-68-0 99%
Reaction Conditions:
Stage #1: 2-Iodothiophenewith diisopropopylaminoborane;triethylamine;triphenylphosphine;palladium dichloride in tetrahydrofuran at 65; for 12 h;Inert atmosphere;Alcaraz-Vaultier borylation;
Stage #2: with methanol in tetrahydrofuran at 0;Inert atmosphere;Further stages;
Steps:
4.2. General procedure for the synthesis of boronic acids from the reaction of aryl iodides, bromides, or triflates with BH2N(iPr)2 in the presence of a palladium catalyst
General procedure: Triphenylphosphene (0.131 g, 0.5 mmol, 20 mol %), p-iodoanisol (0.585 g, 2.5 mmol), and triethylamine (1.78 mL, 12.5 mmol) were added to a 50 mL round-bottomed flask equipped with a sidearm, condenser, and stir bar. This solution was then degassed by alternating vacuum and argon three times. Palladium dichloride (0.023 g, 0.13 mmol, 5 mol %) was then added under positive argon pressure. After stirring at room temperature for 15 min, diisopropylaminoborane (5 mL, 1 M solution in THF, 5 mmol) was added and the reaction mixture was degassed again by alternating vacuum and argon three times. The reaction solution was then heated to reflux. After 12 h of reflux the reaction was cooled to 0 °C and 6 mL of methanol was added through the condenser slowly (Caution: exothermic reaction with evolution of hydrogen). After 15 min of stirring all the solvent was removed under reduced pressure to yield a black solid. This solid was dissolved with sodium hydroxide (3 M, 8 mL) and subsequently washed with hexanes (3×10 mL). The aqueous layer was then cooled to 0 °C (ice bath) and acidified to pH ≤1 with concentrated HCl, with the boronic acid usually precipitating out as a white solid. The aqueous fraction was then extracted with diethyl ether (3×10 mL). The organic fractions were combined, dried with magnesium sulfate and filtered. The solvent was then removed under reduced pressure yielding a white solid.
References:
Haddenham, Dustin;Bailey, Christopher L.;Vu, Chau;Nepomuceno, Gabby;Eagon, Scott;Pasumansky, Lubov;Singaram, Bakthan [Tetrahedron,2011,vol. 67,# 3,p. 576 - 583] Location in patent:experimental part

1003-09-4
484 suppliers
$10.00/25g

6165-68-0
391 suppliers
$14.00/5g
188290-36-0
2 suppliers
inquiry
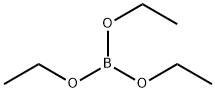
150-46-9
200 suppliers
$14.00/25mL

6165-68-0
391 suppliers
$14.00/5g

1003-09-4
484 suppliers
$10.00/25g

121-43-7
381 suppliers
$14.00/25mL

6165-68-0
391 suppliers
$14.00/5g

1003-09-4
484 suppliers
$10.00/25g

67-56-1
790 suppliers
$9.00/25ml
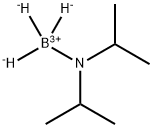
55124-35-1
25 suppliers
$60.00/100mg

6165-68-0
391 suppliers
$14.00/5g