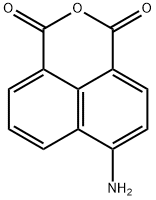
4-Amino-1,8-naphthalic anhydride synthesis
- Product Name:4-Amino-1,8-naphthalic anhydride
- CAS Number:6492-86-0
- Molecular formula:C12H7NO3
- Molecular Weight:213.19
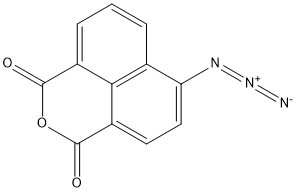
1262149-88-1

6492-86-0
4-Azido-1,8-naphthalenedicarboxylic anhydride (CAS:1262149-88-1, 1.8 g, 7.6 mmol) was used as a raw material and dissolved in 100 mL of acetonitrile. Subsequently, sodium sulfide (6.5 g, 41 mmol) was added to the solution. The reaction mixture was heated to 60 °C and maintained at this temperature for 10 hours. After completion of the reaction, the mixture was cooled to room temperature and then slowly poured into ice water. The precipitate was collected by filtration and dried under vacuum to afford the target product 4-amino-1,8-naphthalenedicarboxylic anhydride as a yellow solid (1.3 g) in 80% yield.
![6-nitro-1H,3H-naphtho[1,8-cd]pyran-1,3-dione](/CAS/GIF/6642-29-1.gif)
6642-29-1
62 suppliers
$22.00/250mg

6492-86-0
121 suppliers
$75.00/100mg
Yield:6492-86-0 98%
Reaction Conditions:
with palladium on activated charcoal;hydrogen in acetonitrile at 20; under 760.051 Torr;
Steps:
Synthesis of 4-amino-naphthalic anhydride
4-nitro-1,8-naphthalic anhydride (1.26 g, 5.20 mmol) was transferredto a three-necked round bottom flask and dissolved in 165 mL ofacetonitrile until the solid completely dissolved to a brown-red solution.Palladium on activated carbon (0.18 g) was added to a three-neckround bottom flask, which was sealed and evacuated. The solutionstirred under H2 for 72 h at room temperature and under atmosphericpressure. The reaction solution was then filtered, resulting in the crudeproduct, which was a dark yellow solid, as well as the catalyst. Thecrude product was refluxed in 250 mL of acetone and filtered hot. Thisprocess was repeated and the aliquots of acetone combined until all ofthe crude product was dissolved in acetone and the palladium catalysthad been removed via filtration. The acetone solution was then filteredthrough Celite and removed via rotary evaporation to yield the purifiedproduct. (yellow solid, 1.11 g, 98%)1H NMR (400 MHz, d6-DMSO) 6.88 (d, J=8 Hz, 1H), 7.69 (t,J=7 Hz, 1H), 7.79 (s, 2H), 8.19 (d, J=8 Hz, 1H), 8.44 (dd, J=1,7 Hz, 1H), 8.70 (dd, J=1,8 Hz, 1H).
References:
Jolley, Elizabeth A.;Hardebeck, Laura K.E.;Ren, Yi;Adams, Miranda S.;Lewis, Michael;Znosko, Brent M. [Biophysical Chemistry,2018,vol. 239,p. 29 - 37]
81-86-7
208 suppliers
$5.00/5g

6492-86-0
121 suppliers
$75.00/100mg
602-87-9
95 suppliers
$16.60/1g

6492-86-0
121 suppliers
$75.00/100mg
83-32-9
276 suppliers
$14.00/5g

6492-86-0
121 suppliers
$75.00/100mg
34087-02-0
79 suppliers
inquiry

6492-86-0
121 suppliers
$75.00/100mg