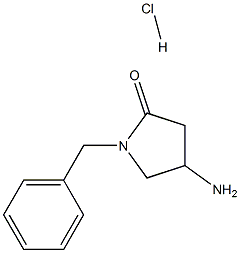
4-AMino-1-benzylpyrrolidin-2-one Hydrochloride synthesis
- Product Name:4-AMino-1-benzylpyrrolidin-2-one Hydrochloride
- CAS Number:478832-05-2
- Molecular formula:C11H15ClN2O
- Molecular Weight:226.7026
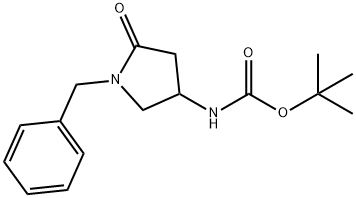
478832-03-0

478832-05-2
1. tert-Butyl 1-benzyl-5-oxopyrrolidin-3-ylcarbamate (480 mg, 1.65 mmol) was dissolved in tetrahydrofuran (6 mL). 2. 4 N hydrogen chloride/dioxane solution (6.0 mL, 24 mmol) was added to the above solution. 3. The reaction mixture was stirred at room temperature overnight. 4. 4. Upon completion of the reaction, diethyl ether (35 mL) was added to the reaction solution and stirring was continued for 30 minutes at room temperature. 5. The precipitate was collected by filtration and washed with ether. 6. 6. 1-Benzyl-4-aminopyrrolidin-2-one hydrochloride (380 mg, 99% yield) was obtained after drying. 7. The product was dried over 1H-NHPA. 7. The product was characterized by 1H-NMR (DMSO-d6): δ 2.23 (1H, dd, J = 4.0, 17.2 Hz), 2.76 (1H, dd, J = 8.6, 17.2 Hz), 3.22 (1H, dd, J = 4.0, 10.8 Hz), 3.53 (1H, dd, J = 7.7, 10.8 Hz), 3.89 ( 1H, bs), 4.30 (1H, d, J = 15.0 Hz), 4.45 (1H, d, J = 15.0 Hz), 7.30 (5H, m), 8.32 (2H, bs).

478832-03-0
25 suppliers
$7.00/250mg

478832-05-2
46 suppliers
$83.00/100mg
Yield:478832-05-2 99%
Reaction Conditions:
with hydrogenchloride in tetrahydrofuran;1,4-dioxane at 20;
Steps:
207.c Example 207; Synthesis of N-(1-benzyl-5-oxopyrrolidin-3-yl)-1H-indazole-5-carboxamide; (c) Synthesis of 4-amino-1-benzyl-2-pyrrolidinone hydrochloride
In tetrahydrofuran (6 ml) was dissolved tert-butyl 1-benzyl-5-oxo-3-pyrrolidinylcarbamate (480 mg, 1.65 mmol), followed by adding thereto 4N-hydrogen chloride/dioxane (6.0 ml, 24 mmol), and the resulting mixture was stirred overnight at room temperature. After completion of the reaction, diethyl ether (35 ml) was added to the reaction solution, and the resulting mixture was stirred at room temperature for 30 minutes and the precipitate was collected by filtration. The precipitate was washed with diethyl ether and dried to obtain 4-amino-1-benzyl-2-pyrrolidinone hydrochloride (380 mg, 99%).1H-NMR (DMSO-d6) δ; 2.23 (1H, dd, J=4.0, 17.2Hz), 2.76 (1H, dd, J=8.6, 17.2Hz), 3.22 (1H, dd, J=4.0, 10.8Hz), 3.53 (1H, dd, J=7.7, 10.8Hz), 3.89 (1H, bs), 4.30 (1H, d, J=15.0Hz), 4.45 (1H, d, J=15.0Hz), 7.30 (5H, m), 8.32 (2H, bs).
References:
EP1403255,2004,A1 Location in patent:Page 79
5733-86-8
154 suppliers
$39.00/1g

478832-05-2
46 suppliers
$83.00/100mg
51535-00-3
136 suppliers
$8.00/1g

478832-05-2
46 suppliers
$83.00/100mg