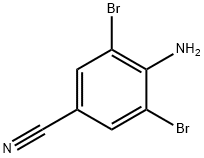
4-Amino-3,5-dibromobenzonitrile synthesis
- Product Name:4-Amino-3,5-dibromobenzonitrile
- CAS Number:58633-04-8
- Molecular formula:C7H4Br2N2
- Molecular Weight:275.93
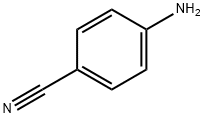
873-74-5

58633-04-8
Preparation of Example 25 4-amino-3,5-dibromobenzonitrile (STR37): To a stirred solution of 100 mg (0.847 mmol) of p-aminobenzonitrile dissolved in 3.6 mL of dioxane was sequentially added 356 μL (1.78 mmol) of 5N sodium hydroxide solution and an appropriate amount (1.78 mmol) of bromine under ice-bath cooling conditions. After removing the ice water bath, the reaction mixture was continued to stir for 1.5 hours. Subsequently, 21.8 μL (0.423 mmol) of bromine was added to ensure that the reaction was complete and stirring was continued for 10 minutes. The reaction mixture was partitioned with ethyl acetate and ice water to separate the organic phase. The organic phase was sequentially washed with brine, dried over anhydrous sodium sulfate, filtered and concentrated. Purification by silica gel plate chromatography using hexane-ethyl acetate (7:3, v/v) as eluent gave 175 mg (74%) of the target product 4-amino-3,5-dibromobenzonitrile. The product was characterized by NMR (CDCl3): δ 5.1 (broad peak, 2H), 7.66 (single peak, 2H).

873-74-5
657 suppliers
$9.00/1g

58633-04-8
71 suppliers
$6.00/250mg
Yield:58633-04-8 175 mg (74%)
Reaction Conditions:
with bromine in 1,4-dioxane;sodium hydroxide;
Steps:
1 Preparation of 4-Amino-3,5-dibromobenzonitrile STR15
EXAMPLE 1 Preparation of 4-Amino-3,5-dibromobenzonitrile STR15 To a stirred solution of 100 mg (0.847 mmoles) of p-aminobenzonitrile in 3.6 mL dioxane chilled in an ice-bath was added sequentially 356 μL (1.78 mmoles) of 5 N sodium hydroxide solution and 284 mg (1.78 mmoles) of bromine. The ice-water bath was removed and the reaction mixture was stirred further for 1.5 hours. After this time, 21.8 μL (0.423 mmoles) of bromine was added to drive the reaction to completion and stirring was continued for 10 minutes. The mixture was partitioned between ethyl acetate and ice-water and the organic phase was separated. It was washed with brine, dried over anhydrous sodium sulfate, filtered, and evaporated. Purification by plate layer chromatography using hexane-ethyl acetate (7:3) as eluant provided 175 mg (74%) of the entitled product. NMR(CDCl3) δ: 5.1 (bs, 2H), 7.66 (s, 2H).
References:
US5192758,1993,A

873-74-5
657 suppliers
$9.00/1g

58633-04-8
71 suppliers
$6.00/250mg