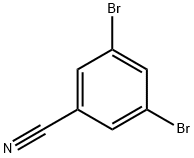
3,5-DIBROMOBENZONITRILE synthesis
- Product Name:3,5-DIBROMOBENZONITRILE
- CAS Number:97165-77-0
- Molecular formula:C7H3Br2N
- Molecular Weight:260.91
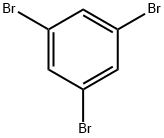
626-39-1

97165-77-0
General procedure for the synthesis of 3,5-dibromobenzonitrile from 1,3,5-tribromobenzene: iPrMgCl (2M in THF, 4.1 mL) and THF (5 mL) were added to a flask containing dry LiCl (0.35 g, 8.24 mmol) at 15 °C. After stirring for 15 min, a solution of 3-bromobenzonitrile (1.46 g, 8.03 mmol) in THF (1 mL) was slowly added to the reaction mixture and stirring was continued for 15 min. Subsequently, DMF (1.3 mL, 12 mmol) was slowly added at 0 °C and the mixture was stirred for 2 hours. After completion of the reaction, aqueous NH3 (7 mL, 28-30%) and I2 (4.06 g, 16 mmol) were added to the mixture and stirred for 2 h at room temperature. The reaction mixture was poured into saturated aqueous Na2SO3 solution and extracted with CHCl3 (3 x 30 mL). The organic layers were combined, dried with Na2SO4 and filtered. After removing the solvent under reduced pressure, the residue was purified by silica gel column chromatography (eluent: hexane/ethyl acetate = 9:1, v/v) to afford pure 3,5-dibromobenzonitrile (0.73 g) in 71% yield. Most of the nitrile compounds involved in this study were commercially available and were identified by comparison with authentic samples.

626-39-1
478 suppliers
$5.00/5g

97165-77-0
165 suppliers
$7.00/1g
Yield:97165-77-0 75%
Reaction Conditions:
Stage #1:1,3,5-trisbromobenzene with isopropylmagnesium chloride;lithium chloride in tetrahydrofuran at -15; for 0.25 h;
Stage #2: with N,N-dimethyl-formamide in tetrahydrofuran at 0; for 2 h;
Stage #3: with ammonia;iodine in tetrahydrofuran;water at 20; for 2 h;
Steps:
3.28 Typical experimental procedure for conversion of aromaticbromides into aromatic nitriles with iPrMgCl, DMF, I2,and aq NH3
General procedure: To a flask containing dried LiCl (0.35 g, 8.24 mmol) was added iPrMgCl (2 M in THF, 4.1 mL) and THF (5 mL) at 15° C. After beingstirred for 15 min, 3-bromo-1-benzonitrile (1.46 g, 8.03 mmol) inTHF (1 mL) was added to the reaction mixture and the obtainedmixture was stirred for 15 min. Then, DMF (1.3 mL, 12 mmol) wasadded at 0° C and the mixture was stirred for 2 h. Then, aq NH3 (7 mL, 28-30%) and I2 (4.06 g, 16 mmol) were added to the reaction mixture. After being stirred for 2 h at room temperature, the reactionmixture was poured into satd aq Na2SO3 solution and was extracted with CHCl3 (3∗30 mL). The organic layer was dried over Na2SO4 and filtered. After removal of the solvent, the residue waspurified by short column chromatography on silica gel (eluent:hexane/ethyl acetate=9:1, v/v) to provide pure 1,3-dicyanobenzene (0.73 g) in 71% yield. Most nitriles mentioned in this work are commercially availableand were identified by comparison with the authentic samples.
References:
Ishii, Genki;Harigae, Ryo;Moriyama, Katsuhiko;Togo, Hideo [Tetrahedron,2013,vol. 69,# 5,p. 1462 - 1469]
145691-59-4
120 suppliers
$6.00/100mg
175205-85-3
67 suppliers
$25.00/1g

97165-77-0
165 suppliers
$7.00/1g
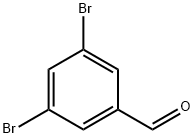
56990-02-4
285 suppliers
$6.00/1g

97165-77-0
165 suppliers
$7.00/1g

175205-85-3
67 suppliers
$25.00/1g

97165-77-0
165 suppliers
$7.00/1g
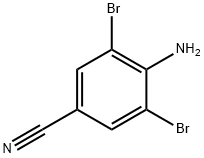
58633-04-8
70 suppliers
$6.00/250mg

97165-77-0
165 suppliers
$7.00/1g