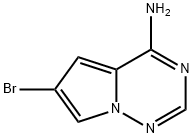
4-AMino-6-broMopyrrolo[1,2-f][1,2,4]triazine synthesis
- Product Name:4-AMino-6-broMopyrrolo[1,2-f][1,2,4]triazine
- CAS Number:937047-06-8
- Molecular formula:C6H5BrN4
- Molecular Weight:213.03
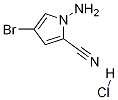
937047-05-7
16 suppliers
inquiry
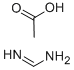
3473-63-0
513 suppliers
$5.00/25g
![4-AMino-6-broMopyrrolo[1,2-f][1,2,4]triazine](/CAS/GIF/937047-06-8.gif)
937047-06-8
43 suppliers
$60.00/5g
Yield: 75%
Reaction Conditions:
with potassium phosphate in ethanol at 78; for 18 h;
Steps:
AAE.5
To a stirred suspension of l-Amino-4-bromo-lH-pyrrole-2-carbonitrile hydrochloride (17 g, 61.1 mmol) in absolute ethanol (350 mL) was added formamidine acetate (31.8 g, 305 mmol) and potassium phosphate (64.9 g, 305 mol). The suspension was heated for 18 hours @ 78 °C (under N2), then cooled, filtered and concentrated to dryness in vacuo. The residue was mixed with ice water (2L) and the dark grayish-brown solids were collected by suction filtration. The solids were taken up in refluxing MeOH and treated with decolorizing carbon, then filtered thru Celite and concentrated dryness in vacuo. The solids were taken up in THF:DCE (1:3) and filtered thru a pad of silica. Removal of the solvent in vacuo provided a yellowish-brown solid. This material was recrystallized from THF:hexanes to provide the desired compound as a yellow solid (9.86 g, 75% yield). (81%). 1H-NMR (DMSO): δ 7.85 (bs, 2H), 7.81 (s, IH), 7.80 (d, IH, J = 2 Hz), 6.96 (d, lH, /= 2 Hz).
References:
BAYER PHARMACEUTICALS CORPORATION WO2007/64883, 2007, A2 Location in patent:Page/Page column 255
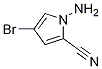
937161-86-9
22 suppliers
inquiry

3473-63-0
513 suppliers
$5.00/25g
![4-AMino-6-broMopyrrolo[1,2-f][1,2,4]triazine](/CAS/GIF/937047-06-8.gif)
937047-06-8
43 suppliers
$60.00/5g