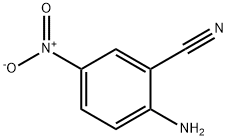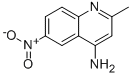
4-AMINO-6-NITRO-QUINALDINE synthesis
- Product Name:4-AMINO-6-NITRO-QUINALDINE
- CAS Number:99185-71-4
- Molecular formula:C10H9N3O2
- Molecular Weight:203.2
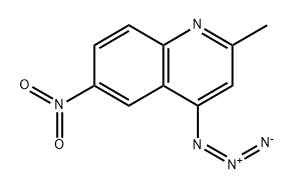
1528735-22-9

99185-71-4
4-Azido-2-methyl-6-nitroquinoline (CAS:1528735-22-9; 29.9 g, 130 mmol) was used as starting material and suspended in THF (450 mL). Subsequently, triphenylphosphine (41.1 g, 160 mmol) was added to the suspension and the resulting mixture was stirred at room temperature for 20 min. Next, water (45 mL) was added and the reaction solution was concentrated to about 200 mL. The concentrated solution was poured into 2.5 M aqueous HCl (1.5 L) and stirred vigorously until the solid was completely dissolved. The aqueous phase was extracted with ether (250 mL x 3), followed by adjusting the pH of the aqueous phase with 50% NaOH aqueous solution to 9. The resulting bright yellow crystalline solid was collected by filtration, washed with cold water (500 mL), and dried to give 2-methyl-6-nitroquinolin-4-amine (25.2 g, 95% yield) as a bright yellow crystalline solid: melting point >250 °C; TLC Rf 0.37 ( Spreading agent ratio 95:4.5:0.5 CHCl3:MeOH:aqueous solution, MeNH2); 1H-NMR (DMSO-d6) δ 9.22 (d, 1H), 8.25 (dd, 1H), 7.77 (d, 1H), 7.30 (s, br, 2H), 6.55 (s, 1H), 2.45 (s, 3H).

1528735-22-9
0 suppliers
inquiry

99185-71-4
32 suppliers
inquiry
Yield:99185-71-4 95%
Reaction Conditions:
with triphenylphosphine in tetrahydrofuran at 20; for 0.333333 h;Inert atmosphere;Staudinger Azide Reduction;
Steps:
4-Amino-2-methyl-6-nitroquinoline (14)
To a suspenion of 4-azido-2-methyl-6-nitroquinoline (13; 29.9 g, 130 mmol) in THF (450 mL) was added triphenylphosphine (41.1 g, 160 mmol). The resulting solution was stirred at room temperature for 20 min and water (45 mL) was added. The solution was evaporated to ~200 mL total volume, then poured into 2.5 M aqueous HCl (1.5 L) and stirred vigorously until the solids had completely dissolved. The aqueous solution was extracted with Et2O (250 mL × 3), and the aqueous solution was adjusted to pH 9 with 50% aqueous NaOH solution. The resulting bright yellow crystalline solid was collected by filtration, washed with cold water (500 mL), and dried to provide 25.2 g (95%) of product as a bright yellow crystalline solid: mp >250 °C; Rf 0.37 (95:4.5:0.5 CHCl3:MeOH:aq. MeNH2); 1H-NMR (DMSO-d6) δ 9.22 (d, 1H), 8.25 (dd, 1h), 7.77 (d, 1H), 7.30 (s, br, 2H), 6.55 (s, 1H), 2.45 (s, 3H).
References:
Williams, John D.;Khan, Atiyya R.;Cardinale, Steven C.;Butler, Michelle M.;Bowlin, Terry L.;Peet, Norton P. [Bioorganic and Medicinal Chemistry,2014,vol. 22,# 1,p. 419 - 434] Location in patent:supporting information
100-01-6
71 suppliers
$11.19/25G

99185-71-4
32 suppliers
inquiry

112219-43-9
4 suppliers
inquiry

99185-71-4
32 suppliers
inquiry
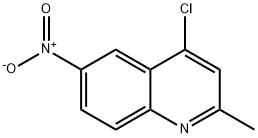
1207-81-4
21 suppliers
inquiry

99185-71-4
32 suppliers
inquiry