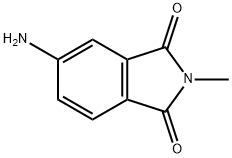
4-AMINO-N-METHYLPHTHALIMIDE synthesis
- Product Name:4-AMINO-N-METHYLPHTHALIMIDE
- CAS Number:2307-00-8
- Molecular formula:C9H8N2O2
- Molecular Weight:176.17
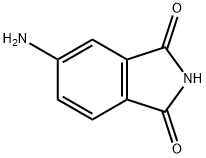
3676-85-5

74-88-4

2307-00-8
GENERAL STEPS: Potassium hydroxide (0.35 g, 6.17 mmol) was added to a solution of 4-amino-phthalimide (1.00 g, 6.17 mmol) in dimethylformamide (30 mL) and the reaction mixture was stirred at room temperature for 2 hours. Subsequently, iodomethane (0.88 g, 6.17 mmol) was added and stirring was continued at the same temperature for 18 hours. Upon completion of the reaction, water (50 mL) and ethyl acetate (50 mL) were added for extraction and the organic phase was separated. The organic phase was washed sequentially with saturated saline, dried with anhydrous sodium sulfate and concentrated under reduced pressure to remove the solvent. The crude product was purified by silica gel column chromatography (elution gradient: 0-100% cyclohexane solution of ethyl acetate) to afford N-methyl-4-amino phthalimide as a yellow solid (1.00 g, 92% yield). The product was characterized as follows: 1H NMR (DMSO-d6) δ 7.46 (d, J = 8.24 Hz, 1H), 6.90 (d, J = 2.00 Hz, 1H), 6.77 (dd, J = 8.24, 2.00 Hz, 1H), 6.42 (br.s, 2H), 2.94 (s, 3H); 13C NMR (DMSO-d6) δ 168.87, 168.57, 155.27, 135.10, 125.17, 117.33, 116.84, 107.42, 23.83; MS (m/z): 177 [M + H]+.

3676-85-5
233 suppliers
$45.00/1g

74-88-4
356 suppliers
$15.00/10g

2307-00-8
191 suppliers
$6.00/250mg
Yield: 92%
Reaction Conditions:
Stage #1:4-aminophthalimide with potassium hydroxide in N,N-dimethyl-formamide at 20; for 2 h;
Stage #2:methyl iodide in N,N-dimethyl-formamide at 20; for 18 h;
Steps:
5-Amino-2-methyl-2,3-dihydro-1H-isoindole-1,3-dione (11a; ZHAWOC3444):
Potassiumhydroxide (0.35 g,6.17 mmol) was added to a solution of 4-aminophthalimide 10 (1.00 g, 6.17 mmol) in dimethylformamide(30 mL) and the mixture was stirred at ambient temperature for 2 h. Iodomethane (0.88 g, 6.17 mmol) wasadded and it was stirred for another 18 h at the same temperature. Water (50 mL) and ethyl acetate (50 mL)was added and the resulting phases were separated. The organic phase was washed with brine, driedover sodium sulfate and concentrated in vacuum. Purification by chromatography on silica gel (Gradient:0-100% ethyl acetate in cyclohexane) afforded the title compound 11a as a yellow solid (1.00 g, 92% yield):1H-NMR (DMSO-d6): δ = 7.46 (d, J = 8.24 Hz, 1H), 6.90 (d, J = 2.00 Hz, 1H), 6.77 (dd, J = 8.24 Hz, 2.00 Hz,1H), 6.42 (br. s, 2H), 2.94 (s, 3H) ppm. 13C-NMR (DMSO-d6): δ = 168.87, 168.57, 155.27, 135.10, 125.17,117.33, 116.84, 107.42, 23.83 ppm. MS (m/z): 177 [M+ H]+.
References:
Fischer, Thomas;Riedl, Rainer [Molecules,2017,vol. 22,# 9]
41663-84-7
286 suppliers
$5.00/5g

2307-00-8
191 suppliers
$6.00/250mg
51832-31-6
35 suppliers
$65.00/100mg

2307-00-8
191 suppliers
$6.00/250mg
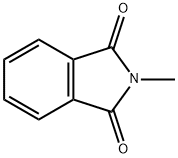
550-44-7
313 suppliers
$20.80/5g

2307-00-8
191 suppliers
$6.00/250mg
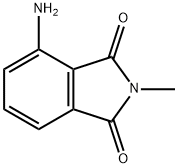
2257-85-4
62 suppliers
$60.00/500 mg
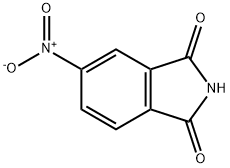
89-40-7
324 suppliers
$5.00/5g

2307-00-8
191 suppliers
$6.00/250mg