
4-(Aminomethyl)-3,5-difluorobenzonitrile synthesis
- Product Name:4-(Aminomethyl)-3,5-difluorobenzonitrile
- CAS Number:633336-81-9
- Molecular formula:C8H6F2N2
- Molecular Weight:168.14

633336-80-8

633336-81-9
The general procedure for the synthesis of 4-(aminomethyl)-3,5-difluorobenzonitrile from the compound (CAS: 633336-80-8) is as follows: to a 20 mL aqueous suspension containing 520 mg 10% Pd/C (50% water), with reference to J. Chem. Soc. Res. (M) (1992) 3128, was added sodium borohydride ( 0.834 g, 0.0221 mol) dissolved in 20 mL of water. Gas release was observed during the reaction. [4-Azadimethyl-2,6-difluorobenzonitrile] (1.26 g, 6.49 mmol; see step (v) above) was dissolved in 50 mL of THF and slowly added to the above aqueous solution over a 15-minute period while cooled in an ice bath. After stirring the reaction mixture for 4 h, 20 mL of 2M HCl was added, followed by filtration through diatomaceous earth. The diatomaceous earth was washed with an appropriate amount of water and the combined aqueous phases were washed with EtOAc and then alkalized with 2M NaOH. The alkalized aqueous phase was extracted three times with dichloromethane and the combined organic phases were washed with water, dried over anhydrous Na2SO4 and concentrated. The product yield was 0.87 g (80%).1H NMR (400 MHz, CDCl3) δ 7.20 (m, 2H), 3.96 (s, 2H), 1.51 (br, 2H).

633336-80-8
0 suppliers
inquiry

633336-81-9
22 suppliers
inquiry
Yield:633336-81-9 80%
Reaction Conditions:
with sodium tetrahydroborate;palladium 10% on activated carbon in tetrahydrofuran;water at 0; for 4.25 h;
Steps:
B.vi Preparation B: Preparation of Compound B; (vi) [4-AMINOMETHVL-2,] [6-DIFLUOROBENZONITRILE]
This reaction was carried out according to the procedure described in J. Chem. Res. (M) (1992) 3128. To a suspension of 520 mg of 10% Pd/C (50% moisture) in 20 mL of water was added a solution of sodium borohydride (0.834 g, 0.0221 mol) in 20 mL of water. Some gas evolution resulted. [4-AZIDOMETHYL-2,] 6- difluorobenzonitrile (1.26 g, 6.49 mmol; see step (v) above) was dissolved in 50 [ML] of THF and added to the aqueous mixture on an ice bath over 15 min. The mixture was stirred for 4 h, whereafter 20 [ML] of 2M HCl was added and the mixture was filtered through Celite. The Celite was rinsed with more water and the combined aqueous phase was washed with EtOAc and subsequently made alkaline with 2M [NAOH.] Extraction three times with methylene chloride followed and the combined organic phase was washed with water, dried [(NA2SO4)] and evaporated. Yield: 0.87 g (80%). [IH] NMR (400 MHz, [CDC13)] [8] 7.20 (m, 2H), 3.96 (s, 2H), 1.51 (broad, 2H)
References:
WO2003/101957,2003,A1 Location in patent:Page 36
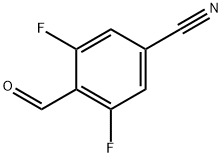
467442-15-5
106 suppliers
$18.00/50mg

633336-81-9
22 suppliers
inquiry
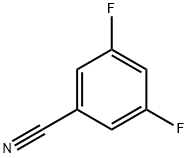
64248-63-1
320 suppliers
$6.00/5g

633336-81-9
22 suppliers
inquiry