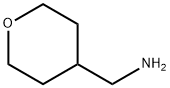
4-(Aminomethyl)tetrahydro-2H-pyran synthesis
- Product Name:4-(Aminomethyl)tetrahydro-2H-pyran
- CAS Number:130290-79-8
- Molecular formula:C6H13NO
- Molecular Weight:115.17
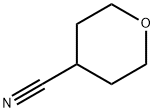
4295-99-2

130290-79-8
Example 43 (Synthesis of 4-aminomethyltetrahydropyran): In a 200 mL stainless steel autoclave equipped with a stirring device, a thermometer, and a manometer, 10.0 g (90.0 mmol) of 4-cyanotetrahydropyran, 50.0 g of a 22% (w/w) ammonia-methanol solution, and 2.0 g (17.0 mmol as nickel atoms) of activated Raney nickel ( provided by Nikki Chemical Co., Ltd; nickel sponge N154D). The reaction was stirred under hydrogen atmosphere at 45 to 55 °C and 0.51 to 0.61 MPa pressure for 17 hours. After completion of the reaction, the insoluble material was removed by filtration and the filtrate was washed with 30 mL of methanol. The filtrate and washings were combined, concentrated under reduced pressure and then distilled under reduced pressure at 73-74 °C and 2.67 kPa to give 7.94 g (76.6% isolated yield) of 4-aminomethyltetrahydropyran as a colorless liquid. Its physical properties were as follows: 1H-NMR (DMSO-d6, δ (ppm)): 1.02-1.16 (2H, m), 1.10-1.50 (2H, brs), 1.34-1.45 (1H, m), 1.56-1.61 (2H, m), 2.39 (2H, d, J = 6.3 Hz), 3.20-3.29 (2H, m ), 3.81-3.86 (2H, m); CI-MS (m/e): 116 (M+1), 99. Example 45 (Synthesis of 4-aminomethyltetrahydropyran): in a 200 mL stainless steel autoclave equipped with a stirring device, a thermometer, and a manometer, 10.0 g (90.0 mmol) of 4-cyanotetrahydropyran, 100 mL of a 22% (w/w) ammonia-methanol solution, and 2.0 g (17.0 mmol as nickel atoms) of activated Raney nickel ( provided by Nikki Chemical Co., Ltd; nickel sponge N154D). The reaction was stirred under hydrogen atmosphere at 50 to 60 °C and 0.51 to 0.61 MPa pressure for 5 hours. After completion of the reaction, the insoluble material was removed by filtration and the filtrate was washed with 30 mL of methanol. The filtrate and washings were combined and analyzed by gas chromatography (internal standard method) to confirm the formation of 8.84 g (85.3% reaction yield) of 4-aminomethyltetrahydropyran and no by-product bis(4-tetrahydropyranosylmethyl)amine was detected.

4295-99-2
168 suppliers
inquiry

130290-79-8
307 suppliers
$6.00/1g
Yield:130290-79-8 85.3%
Reaction Conditions:
with ammonia;hydrogen;Raney nickel in methanol at 45 - 60; under 3825.38 - 4575.46 Torr; for 5 - 17 h;Product distribution / selectivity;
Steps:
43; 45 Example 43
Example 43 (Synthesis of 4-aminomethyltetrahydropyran) In an autoclave made of stainless equipped with a stirring device, a thermometer and a pressure gauge and having an inner volume of 200 ml were charged 10.0 g (90.0 mmol) of 4-cyanotetrahydropyran, 50.0 g of 22% by weight ammonia-methanol solution and 2.0 g (17.0 mmol in terms of a nickel atom) of developed Raney nickel (available from Nikki Chemical Co., Ltd.; sponge nickel N154D), and the mixture was reacted under hydrogen atmosphere (0.51 to 0.61MPa) at 45 to 55°C for 17 hours under stirring. After completion of the reaction, insoluble materials were filtered, and the filtrated material was washed with 30 ml of methanol. The filtrate and the washed solution were combined and concentrated under reduced pressure, and then, the concentrate was distilled under reduced pressure (73 to 74°C, 2.67 kPa) to tive 7.94 g (Isolation yield; 76.6%) of 4-aminomethyltetrahydropyran as colorless liquid. Physical properties of the 4-aminomethyltetrahydropyran are as follows. 1H-NMR (DMSO-d6, δ (ppm)); 1.02 to 1.16 (2H, m), 1.10 to 1.50 (2H, brs), 1.34 to 1.45 (1H, m), 1.56 to 1.61 (2H, m), 2.39 (2H, d, J=6.3Hz), 3.20 to 3.29 (2H, m), 3.81 to 3.86 (2H, m) CI-MS (m/e); 116 (M+1), 99 Example 45 (Synthesis of 4-aminomethyltetrahydropyran) In an autoclave made of stainless equipped with a stirring device, a thermometer and a pressure gauge and having an inner volume of 200 ml were charged 10.0 g (90.0 mmol) of 4-cyanotetrahydropyran, 100 ml of 22% by weight ammonia methanol solution and 2.0 g (17.0 mmol in terms of a nickel atom) of developed Raney nickel (available from Nikki Chemical Co., Ltd.; sponge nickel N154D), and the mixture was reacted under hydrogen atmosphere (0.51 to 0.61MPa) at 50 to 60°C for 5 hours under stirring. After completion of the reaction, insoluble materials were filtered, the filtered material was washed with 30 ml of methanol, and the filtrate and the washed solution were combined. When this solution was analyzed by gas chromatography (Internal standard method), 8.84 g (Reaction yield: 85.3%) of 4-aminomethyltetrahydropyran was found to be formed. Incidentally, bis(4-tetrahydropyranylmethyl)amine which is a by-product was not formed.
References:
Ube Industries, Ltd. EP1671937, 2006, A1 Location in patent:Page/Page column 16-17
344329-76-6
122 suppliers
$7.00/250mg

130290-79-8
307 suppliers
$6.00/1g
362703-30-8
61 suppliers
inquiry

130290-79-8
307 suppliers
$6.00/1g
85064-61-5
155 suppliers
$6.00/100mg

130290-79-8
307 suppliers
$6.00/1g
125552-89-8
226 suppliers
$11.00/1g

130290-79-8
307 suppliers
$6.00/1g