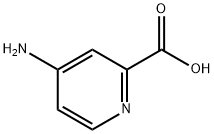
4-Aminopyridine-2-carboxylic acid synthesis
- Product Name:4-Aminopyridine-2-carboxylic acid
- CAS Number:100047-36-7
- Molecular formula:C6H6N2O2
- Molecular Weight:138.12
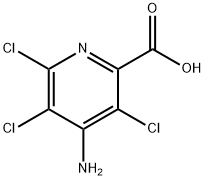
1918-02-1

100047-36-7
General procedure for the synthesis of 4-aminopyridine-2-carboxylic acid from 4-amino-3,5,6-trichloropyridine-2-carboxylic acid: a thick suspension formed by Picloram (8 g, 33 mmol) and 10% Pd/C (1.2 g) in a 10% aqueous LiOH solution (44 mL) was purged twice with hydrogen, and then under a hydrogen atmosphere (45 PSI) at 40°C for 4 hours with stirring. Subsequently, the mixture was heated to 70°C and maintained for 12 hours. Upon completion of the reaction, the cooled suspension was filtered through diatomaceous earth. The filtrate was acidified to pH=3 in concentrated HCl (~3.5 mL), at which point a precipitate was generated. The solid product was collected by filtration and dried overnight under high vacuum to give the final 4-aminopyridine-2-carboxylic acid as a beige solid (4.6 g, 99% yield).LCMS analysis showed m/z 139.1 (M + H)+ with a retention time of 0.21 minutes.

1918-02-1
291 suppliers
$14.14/1gm:

100047-36-7
196 suppliers
$18.00/250mg
Yield:100047-36-7 99%
Reaction Conditions:
Stage #1:picloram with hydrogen;lithium hydroxide;palladium 10% on activated carbon in water at 40 - 70; under 2327.23 Torr; for 16 h;
Stage #2: with hydrogenchloride in water; pH=3
Steps:
38.38A
Intermediate 38A 4-aminopicolinic acid A thick suspension of Picloram (8g, 33 mmol) and 10% Pd / C (1.2g) in a 10% LiOH aqueous solution (44 mL) was purged with hydrogen gas twice then stirred under a hydrogen atmosphere (45 PSI) at 40°C for 4 hours. The mixture was then heated to 70°C for 12 hours. The cooled suspension was filtered on Celite. The filtrate was made acidic (pH = 3) with concentrated HCI (app. 3.5 mL) at wich point a precipitate formed. The solid was filtered off and dried under Hi-Vac overnight to afford the title compound as a beige solid (4.6g, 99%). LCMS m/z 139.1 (M + H)+, ret. time= 0.21 min.
References:
UNIVERSITÉ DE MONTRÉAL;RUEL, Réjean;CHANTIGNY, Yves;MARINIER, Anne;RENÉ, Patricia;BOUVIER, Michel WO2012/3576, 2012, A1 Location in patent:Page/Page column 96
14933-78-9
35 suppliers
inquiry

100047-36-7
196 suppliers
$18.00/250mg
18437-58-6
326 suppliers
$6.00/1g

100047-36-7
196 suppliers
$18.00/250mg
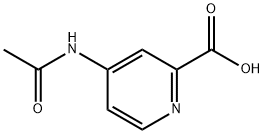
84487-16-1
27 suppliers
$251.00/1g

100047-36-7
196 suppliers
$18.00/250mg
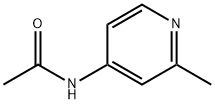
18085-47-7
14 suppliers
inquiry

100047-36-7
196 suppliers
$18.00/250mg