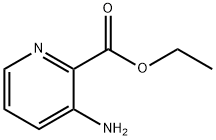
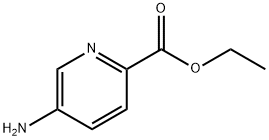
3-Aminopyridine-2-carboxylic acid ethyl ester synthesis
- Product Name:3-Aminopyridine-2-carboxylic acid ethyl ester
- CAS Number:27507-15-9
- Molecular formula:C8H10N2O2
- Molecular Weight:166.18

64-17-5
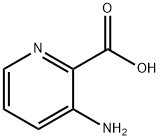
1462-86-8

27507-15-9
3-Aminopyridine-2-carboxylic acid (28 g, 200 mmol, 1.0 eq.) was suspended in ethanol (800 mL) at room temperature and bubbled through hydrogen chloride gas (HCl(g)) for 10 min, and the reaction mixture was transformed into a clarified yellow solution. The reaction mixture was heated to 100 °C and after stirring the reaction for 1 day, LCMS analysis showed that about 50% of the feedstock was unreacted. The reaction mixture was cooled to room temperature, bubbled again with hydrogen chloride gas (HCl(g)) for 10 minutes, and then reheated to 100 °C. The reaction mixture was then stirred for 1 day and the LCMS analysis showed that about 50% of the feedstock was unreacted. After continuing to stir the reaction for 1 day, LCMS analysis still showed that about 50% of the feedstock was unreacted. The reaction mixture was cooled to room temperature and concentrated under reduced pressure to remove all solvent. The residue was resuspended in ethanol (800 mL) and bubbled through hydrogen chloride gas (HCl(g)) for 10 minutes. The resulting mixture was reacted at reflux in an oil bath at 100 °C for 1 day.LCMS analysis showed a conversion of about 70-75%. The reaction mixture was cooled to room temperature and concentrated under reduced pressure to remove all solvents. The solid residue was dissolved in water (250 mL) and the pH was adjusted to 8-9 with saturated aqueous sodium carbonate (Na2CO3) for bursting. Gas production and formation of a white solid was observed. The suspension was filtered, the solid was washed with water and dried under vacuum at 65 °C to give ethyl 3-aminopyridine-2-carboxylate (22 g, 65% yield) as a white solid.LCMS (APCI, M++1) 167.0; 1H NMR (300 MHz, DMSO-d6) δ ppm 7.84 (dd, J=4.05,1.60 Hz, 1H), and 7.23-7.30 (m, 1H), 7.16-7.23 (m, 1H), 6.65 (br.s., 2H), 4.27 (q, J=7.10 Hz, 2H), 1.30 (t, J=7.06 Hz, 3H).

64-17-5
825 suppliers
$10.00/50g

1462-86-8
257 suppliers
$10.00/1g

27507-15-9
114 suppliers
$11.00/250mg
Yield:27507-15-9 65%
Reaction Conditions:
with hydrogenchloride at 20 - 100; for 72 h;
Steps:
Step 1 - Synthesis of ethyl 3-aminopicolinate (C-2)
A suspension of 3-aminopicolinic acid (1-18) (28 g, 200 mmoL, 1.0 eq) in EtOH (800 mL) at room temperature was bubbled with HCI (g) for 10 min. The reaction turned into a clear yellow solution. After being stirred at 100°C for 1 day, LCMS indicated that about 50% of starting material remained. The reaction mixture was cooled to rt and bubbled with HCI (g) for 10 min. The mixture was then re-heated to 100 °C. After being stirred at 100 °C for 1 day, LCMS indicated that about 50% of the starting material remained. The reaction mixture was cooled to rt and all solvents were removed under reduced pressure. The residue was resolved in EtOH (800 mL) and was bubbled with HCI (g) for 10 min. The resulting mixture was refluxed at 100°C oil bath for 1 day. LCMS indicated about 70-75 % conversion. The reaction mixture was cooled to rt and all solvents were removed under reduced pressure. The solid was dissolved in 250 mL water and the solution was quenched to pH = 8-9 with saturated aq Na2C03. A gas was generated and a white solid formed. The suspension was filtered, washed with water, and dried under vacuum at 65°C to afford 22 g of ethyl 3-aminopicolinate (1-19) in 65% yleld as a white solid. LCMS (APCI, M+ + 1 ) 167.0; 1H NMR (300 MHz, DMSO-c/6) 8 ppm 7.84 (dd, J = 4.05, 1 .60 Hz, 1 H), 7.23 - 7.30 (m, 1 H), 7.16 - 7.23 (m, 1 H), 6.65 (br. s., 2 H), 4.27 (q, J = 7.10 Hz, 2 H), 1 .30 (t, J = 7.06 Hz, 3 H).
References:
WO2016/97918,2016,A1 Location in patent:Page/Page column 55
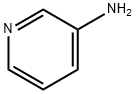
462-08-8
476 suppliers
$14.00/25g
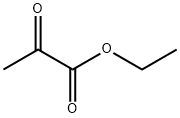
617-35-6
564 suppliers
$5.00/10g

27507-15-9
114 suppliers
$11.00/250mg
119830-47-6
53 suppliers
$45.00/10mg

119830-48-7
0 suppliers
inquiry