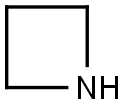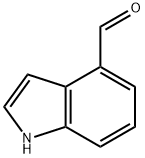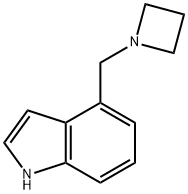
4-(Azetidin-1-ylMethyl)-1H-indole synthesis
- Product Name:4-(Azetidin-1-ylMethyl)-1H-indole
- CAS Number:1001395-40-9
- Molecular formula:C12H14N2
- Molecular Weight:186.25
Yield:1001395-40-9 52%
Reaction Conditions:
Stage #1: azetidine;4-formyl indolewith sodium tris(acetoxy)borohydride in tetrahydrofuran; for 1 h;
Stage #2: with water;sodium hydrogencarbonate in tetrahydrofuran;dichloromethane;
Steps:
77
Intermediate 77; 4-(Azetidin-l-ylmethyl)-lH-indole; Sodium triacetoxy borohydride (1.46 g, 6.9 mmol) was added to a solution of lH-indole-4- carbaldehyde (0.5 g, 3.4 mmol) and azetidine (0.39 g, 6.87 mmol) in THF (15 ml). The mixture was stirred for Ih and diluted with dichloromethane and NaHCCβ (aq). The organic phase was washed with brine (Ix), dried (MgSO4) and evaporated. The crude product was dissolved in dichloromethane and hexane was added (1:1). The offwhite powder was filtered and washed with a mixture of dichloromethane hexane (1:1). Yield: 400 mg (52%). Offwhite solid. MS (ESI+) for Ci2Hi4N2 m/z 187 (M+H)+.
References:
WO2008/3703,2008,A1 Location in patent:Page/Page column 124-125