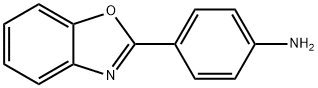
4-BENZOOXAZOL-2-YL-PHENYLAMINE synthesis
- Product Name:4-BENZOOXAZOL-2-YL-PHENYLAMINE
- CAS Number:20934-81-0
- Molecular formula:C13H10N2O
- Molecular Weight:210.23
Yield:20934-81-0 92%
Reaction Conditions:
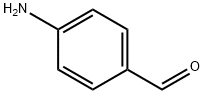
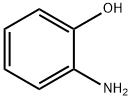
Stage #1: para-aminobenzaldehyde;2-amino-phenol in ethanol; for 3 h;Reflux;
Stage #2: with lead (IV) acetate in acetic acid at 20; for 1 h;
Steps:
4-(1,3-Benzoxazol-2-yl)aniline (6).
The mixture of 2-aminophenol 4 (10 g, 91.63 mmol) with 4-aminobenzaldehyde 5 (11.3 g, 91.63 mmol) was refluxed in10 mL of ethanol for 3 h. After cooling down the reaction mixture, ethanol was removed in vacuum. Theresulting Schiff base was dissolved in 12 mL of acetic acid, and lead tetraacetate (40 g, 91.63 mmol) was added. The mixture was stirred at room temperature for 1 h, then diluted with 30 mL H2O, extracted with ethyl acetate, and dried over anhydrous Na2SO4. The solvent was removed in vacuum, and the crude product was purified by column chromatography with ethylacetate/hexane (3 : 7) to afford pure compound 6.Yield 92%, mp 359-361°C. 1H NMR spectrum, δ,ppm: 5.97 s (2H), 6.57- 6.88 m (2H), 7.30 d.d.d (2H,J = 6.4, 5.4, 3.7 Hz), 7.65 d.d (2H, J = 6.4, 2.6 Hz),7.76 -7.98 m (2H). MS (ESI): 211 [M + H]+.
References:
Ravikumar;Raolji;Venkata Sastry;Kalidasu;Balaaraju [Russian Journal of General Chemistry,2018,vol. 88,# 10,p. 2183 - 2189]
![2-(4-bromophenyl)benzo[d]oxazole](/CAS/GIF/3164-13-4.gif)
3164-13-4
154 suppliers
$16.00/250mg

20934-81-0
122 suppliers
$133.00/5G
273-53-0
205 suppliers
$10.00/5g

106-40-1
484 suppliers
$12.00/5g

20934-81-0
122 suppliers
$133.00/5G
2835-68-9
474 suppliers
$5.00/5g
583-53-9
324 suppliers
$10.00/5g

20934-81-0
122 suppliers
$133.00/5G