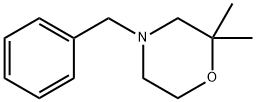
4-benzyl-2,2-dimethylmorpholine synthesis
- Product Name:4-benzyl-2,2-dimethylmorpholine
- CAS Number:84761-04-6
- Molecular formula:C13H19NO
- Molecular Weight:205.3
![2-[Benzyl(2-Methylprop-2-En-1-Yl)Amino]Ethan-1-Ol](/CAS/20180703/GIF/892871-67-9.gif)
892871-67-9

84761-04-6
Mercury(II) acetate (20.7 g, 65.0 mmol) was added as 2-[benzyl-(2-methyl-allyl)-amino]-ethanol (13.0 g, 63.2 mmol), which was dissolved in a mixed solvent of water (45 mL) and THF (45 mL). After 3 hours of reaction, the reaction mixture was treated with 2.5 M NaOH aqueous solution (25 mL, 125 mmol) followed by the addition of NaBH4 (2.72 g, 71.9 mmol). After 19 h of reaction, the mixture was decanted from the metallic mercury and transferred to a partition funnel containing tert-butyl methyl ether (250 mL). The organic phase was separated, washed with water (250 mL), filtered through a silica gel plug and the filtrate concentrated. The residue was purified by fast chromatography using a gradient elution of 5% to 10% tert-butyl methyl ether in CH2Cl2. The grades containing 4-benzyl-2,2-dimethylmorpholine were collected, concentrated, dissolved in hexane (100 mL) and filtered through Celite. The filtrate was concentrated to give 4-benzyl-2,2-dimethylmorpholine (7.01 g, 54% yield) as a colorless liquid.1H NMR (DMSO-d6, 400 MHz) δ 7.20-7.40 (5H, m), 3.60 (2H, m), 3.42 (2H, s), 2.29 (2H, m), 2.10 (2H, s), 1.14 ( 6H, s).
![2-[Benzyl(2-Methylprop-2-En-1-Yl)Amino]Ethan-1-Ol](/CAS/20180703/GIF/892871-67-9.gif)
892871-67-9
12 suppliers
$175.00/100mg

84761-04-6
26 suppliers
$101.00/100mg
Yield:84761-04-6 54%
Reaction Conditions:
Stage #1: 2-(benzyl(2-methylallyl)amino)ethanolwith mercury(II) diacetate in tetrahydrofuran;water; for 3 h;
Stage #2: with sodium hydroxide in tetrahydrofuran;water; for 19 h;
Steps:
151
Add 2-[benzyl-(2-methyl-allyl)-amino]-ethanol (13.0 g, 63.2 mmol) to a slurry of mercury (H) acetate (20.7 g, 65.0 mmol) in water (45 mL) and THF (45 mL). After 3 h, treat the mixture with NaOH (25 mL, 2.5 M aqueous, 125 mmol) followed by NaBH4 (2.72 g, 71.9 mmol). After 19 h, decant the mixture away from the metallic mercury and add to a separatory funnel with fert-butyl methyl ether (250 mL). Separate the organic solution, wash with water (250 mL), filter through a silica plug, and concentrate. Purify the residue by flash chromatography using a gradient from 5% to 10% tert-butyl methyl ether in CH2Cl2. Collect and concentrate the fractions containing product then dissolve the residue in hexanes (100 mL). Filter the solution through Celite to remove metallic mercury and then concentrate the filtrate to give the title compound (7.01g, 54%) as a colorless liquid. 1H NMR (DMSO-d6, 400 MHz) δ 7.20-7.40 (5H, m), 3.60 (2H, m), 3.42 (2H, s), 2.29 (2H. m), 2.10 (2H, s), 1.14 (6H, s).
References:
WO2006/66174,2006,A1 Location in patent:Page/Page column 132
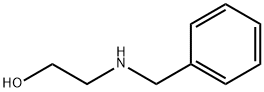
104-63-2
360 suppliers
$7.00/10g

84761-04-6
26 suppliers
$101.00/100mg